Inter-laboratory assessment of PrPSc typing in creutzfeldt-jakob disease: a Western blot study within the NeuroPrion Consortium - PubMed (original) (raw)
Multicenter Study
doi: 10.1111/j.1750-3639.2008.00187.x. Epub 2008 Jul 2.
Silvio Notari, Petra Weber, Heinz Schimmel, Herbert Budka, Isidre Ferrer, Stéphane Haik, Jean-Jacques Hauw, Mark W Head, James W Ironside, Lucia Limido, Agustin Rodriguez, Thomas Ströbel, Fabrizio Tagliavini, Hans A Kretzschmar
Affiliations
- PMID: 18624793
- PMCID: PMC8094660
- DOI: 10.1111/j.1750-3639.2008.00187.x
Multicenter Study
Inter-laboratory assessment of PrPSc typing in creutzfeldt-jakob disease: a Western blot study within the NeuroPrion Consortium
Piero Parchi et al. Brain Pathol. 2009 Jul.
Abstract
Molecular typing is of considerable importance for the surveillance and epidemiology of human transmissible spongiform encephalopathies (TSEs). It relies on the detection of distinct protease-resistant prion protein (PrP(Sc)) core fragments that differ in molecular mass and/or glycoform ratio. In this collaborative study, we tested the inter-laboratory agreement in TSE molecular typing. Sixteen characterized brain specimens from sporadic TSEs and variant Creutzfeldt-Jakob disease (vCJD) cases were distributed blindly to seven laboratories for molecular characterization by a defined protocol and classification. Agreement between laboratories in the classification of samples was excellent. In particular, there were no differences in the distinction between PrP(Sc) type 1, type 2A, and type 2B with one exception, which eventually was identified as a case with types 1 and 2 co-occurrence. This shows that the general technique and particular classification system used here are robust and represent a reliable basis for diagnostic and epidemiologic purposes. The subtle further distinction of subtypes among type 1 and type 2 groups requires high-sensitivity gel electrophoresis protocols that are unsuitable for routine diagnostic needs and must be reserved for research investigations. Further research is necessary on the identification and significance of co-occurrence of PrP(Sc) types 1 and 2 within one brain.
Figures
Figure 1
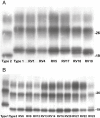
Immunoblot analysis of PrPSc extracted from frontal cortex homogenates of blindly coded human TSE brain samples. All samples were treated according to given instructions (see Materials and Methods) and were separated in a conventional gel electrophoresis system [12% Tris–glycine sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) with 6.5‐cm separation distance]. Panel A shows an example of the first analyses, while panel B illustrate an example of the second analyses, during which all cases identified as having the same PrPSc type were run together.
Figure 2
Immunoblot analysis of PrPSc extracted from frontal cortex homogenates of blindly coded human TSE brain samples. All samples were treated according to given instructions (see Materials and Methods) and were separated in a high‐resolution gel electrophoresis system [15% Tris–gylcine sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE) with 15‐cm separation distance]. The samples, previously analyzed in a conventional gel electrophoresis system (see Figure 1), were diluted or concentrated to even the signal intensities of the samples and were loaded either onto gel 1 (A) or gel 2 (B) according to their migration pattern (ie, type 1 or type 2) determined in the conventional gel electrophoresis system.
Figure 3
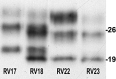
Western blot analyses (laboratory 4) on case RV18 showing a co‐occurrence of PrPSc types 1 and 2. One PrPSc type 1 (RV17) and two PrPSc type 2 samples (RV22 and RV23) are included for comparison.
Similar articles
- Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease.
Casalone C, Zanusso G, Acutis P, Ferrari S, Capucci L, Tagliavini F, Monaco S, Caramelli M. Casalone C, et al. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3065-70. doi: 10.1073/pnas.0305777101. Epub 2004 Feb 17. Proc Natl Acad Sci U S A. 2004. PMID: 14970340 Free PMC article. - Classification of sporadic Creutzfeldt-Jakob disease revisited.
Cali I, Castellani R, Yuan J, Al-Shekhlee A, Cohen ML, Xiao X, Moleres FJ, Parchi P, Zou WQ, Gambetti P. Cali I, et al. Brain. 2006 Sep;129(Pt 9):2266-77. doi: 10.1093/brain/awl224. Brain. 2006. PMID: 16923954 - A refined method for molecular typing reveals that co-occurrence of PrP(Sc) types in Creutzfeldt-Jakob disease is not the rule.
Notari S, Capellari S, Langeveld J, Giese A, Strammiello R, Gambetti P, Kretzschmar HA, Parchi P. Notari S, et al. Lab Invest. 2007 Nov;87(11):1103-12. doi: 10.1038/labinvest.3700676. Epub 2007 Sep 24. Lab Invest. 2007. PMID: 17893675 - Neuropathology and molecular biology of variant Creutzfeldt-Jakob disease.
Ironside JW, Head MW. Ironside JW, et al. Curr Top Microbiol Immunol. 2004;284:133-59. doi: 10.1007/978-3-662-08441-0_6. Curr Top Microbiol Immunol. 2004. PMID: 15148991 Review. - Pathological diagnosis of variant Creutzfeldt-Jakob disease.
Ironside JW, McCardle L, Horsburgh A, Lim Z, Head MW. Ironside JW, et al. APMIS. 2002 Jan;110(1):79-87. doi: 10.1034/j.1600-0463.2002.100110.x. APMIS. 2002. PMID: 12064259 Review.
Cited by
- Incidence and spectrum of sporadic Creutzfeldt-Jakob disease variants with mixed phenotype and co-occurrence of PrPSc types: an updated classification.
Parchi P, Strammiello R, Notari S, Giese A, Langeveld JP, Ladogana A, Zerr I, Roncaroli F, Cras P, Ghetti B, Pocchiari M, Kretzschmar H, Capellari S. Parchi P, et al. Acta Neuropathol. 2009 Nov;118(5):659-71. doi: 10.1007/s00401-009-0585-1. Epub 2009 Aug 29. Acta Neuropathol. 2009. PMID: 19718500 Free PMC article. - The characterization of AD/PART co-pathology in CJD suggests independent pathogenic mechanisms and no cross-seeding between misfolded Aβ and prion proteins.
Rossi M, Kai H, Baiardi S, Bartoletti-Stella A, Carlà B, Zenesini C, Capellari S, Kitamoto T, Parchi P. Rossi M, et al. Acta Neuropathol Commun. 2019 Apr 8;7(1):53. doi: 10.1186/s40478-019-0706-6. Acta Neuropathol Commun. 2019. PMID: 30961668 Free PMC article. - Revisiting the Heidenhain Variant of Creutzfeldt-Jakob Disease: Evidence for Prion Type Variability Influencing Clinical Course and Laboratory Findings.
Baiardi S, Capellari S, Ladogana A, Strumia S, Santangelo M, Pocchiari M, Parchi P. Baiardi S, et al. J Alzheimers Dis. 2016;50(2):465-76. doi: 10.3233/JAD-150668. J Alzheimers Dis. 2016. PMID: 26682685 Free PMC article. - Epidemiological characteristics of human prion diseases.
Chen C, Dong XP. Chen C, et al. Infect Dis Poverty. 2016 Jun 2;5(1):47. doi: 10.1186/s40249-016-0143-8. Infect Dis Poverty. 2016. PMID: 27251305 Free PMC article. Review. - The Three Glycotypes in the London Classification System of Sporadic Creutzfeldt-Jakob Disease Differ in Disease Duration.
Ney B, Eratne D, Lewis V, Ney L, Li QX, Stehmann C, Collins S, Velakoulis D. Ney B, et al. Mol Neurobiol. 2021 Aug;58(8):3983-3991. doi: 10.1007/s12035-021-02396-9. Epub 2021 Apr 26. Mol Neurobiol. 2021. PMID: 33904020
References
- Baron T, Biacabe AG, Arsac JN, Benestad S, Groschup MH (2007) Atypical transmissible spongiform encephalopathies (TSEs) in ruminants. Vaccine 25:5625–5630. - PubMed
- Brown P, Coker‐Vann M, Pomeroy K, Franko M, Asher DM, Gibbs CJ Jr, Gajdusek DC (1986) Diagnosis of Creutzfeldt–Jakob disease by Western blot identification of marker protein in human brain tissue. N Engl J Med 314:547–551. - PubMed
- Bruce ME, McConnell I, Fraser H, Dickinson AG (1991) The disease characteristics of different strains of scrapie in Sinc congenic mouse lines: implications for the nature of the agent and host control of pathogenesis. J Gen Virol 72:595–603. - PubMed
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A et al (1997) Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389:498–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials