Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells - PubMed (original) (raw)
Comparative Study
Activation of beta-catenin signalling by GSK-3 inhibition increases p-glycoprotein expression in brain endothelial cells
Joseph C Lim et al. J Neurochem. 2008 Aug.
Abstract
This study investigates involvement of beta-catenin signalling in regulation of p-glycoprotein (p-gp) expression in endothelial cells derived from brain vasculature. Pharmacological interventions that enhance or that block beta-catenin signalling were applied to primary rat brain endothelial cells and to immortalized human brain endothelial cells, hCMEC/D3, nuclear translocation of beta-catenin being determined by immunocytochemistry and by western blot analysis to confirm effectiveness of the manipulations. Using the specific glycogen synthase kinase-3 (GSK-3) inhibitor 6-bromoindirubin-3'-oxime enhanced beta-catenin and increased p-gp expression including activating the MDR1 promoter. These increases were accompanied by increases in p-gp-mediated efflux capability as observed from alterations in intracellular fluorescent calcein accumulation detected by flow cytometry. Similar increases in p-gp expression were noted with other GSK-3 inhibitors, i.e. 1-azakenpaullone or LiCl. Application of Wnt agonist [2-amino-4-(3,4-(methylenedioxy) benzylamino)-6-(3-methoxyphenyl)pyrimidine] also enhanced beta-catenin and increased transcript and protein levels of p-gp. By contrast, down-regulating the pathway using Dickkopf-1 or quercetin decreased p-gp expression. Similar changes were observed with multidrug resistance protein 4 and breast cancer resistance protein, both known to be present at the blood-brain barrier. These results suggest that regulation of p-gp and other multidrug efflux transporters in brain vasculature can be influenced by beta-catenin signalling.
Figures
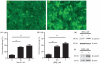
Fig. 1
Effect of pan Wnt agonist and GSK-3β inhibitor, BIO on stabilization and localization of β-catenin in human brain endothelial cells. Staining for β-catenin is shown in (a) untreated and (b) cells treated with 1 μM BIO for 16 h. The percentage of cells showing nuclear/perinuclear staining for β-catenin 16 h following treatment with (c) pan Wnt agonist or (d) GSK-3β inhibitor, BIO at the concentrations shown was calculated relative to the total number of DAPI-stained nuclei; in each case, values shown as the mean ± SEM are significantly higher than those of untreated control cells (**p < 0.01, n = 5). (e and f) Western blots of (e) whole cell lysates and (f) nuclear fractions from untreated cells and from cells treated with 1 μM BIO for 16 h showing increased intensity of the band corresponding to β-catenin in the treated cells. In each case, the blot is representative of immunoblots resulting from three separate experiments.
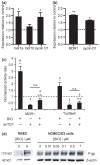
Fig. 2
Effect of GSK-3β inhibitor, BIO, on p-gp expression in (a) rat brain endothelial cells and (b) human immortalized brain endothelial cells. Cells were exposed to 1 μM BIO for 16 h (hCMEC/D3) or to 5 μM BIO for 5 days (rat) and then harvested for analysis at transcript level (a and b) by qRT-PCR. Expression was firstly estimated relative to the geometric mean of three most stable reference genes using
ge
N
orm
analysis. Values shown are the mean ± SEM of these amounts expressed relative to the expression in control untreated cells for mdr1a or MDR1, mdr1b and cyclin D1. Significant increases over control are shown (a) for mdr1a (*p < 0.05, n = 6), cyclin D1 (*p < 0.05, n = 6) but not mdr1b in the rat cells and (b) for MDR1 (**p < 0.01, n = 4) and cyclin D1 (*p < 0.05, n = 4) in the human cells. (c) Activation of MDR1 transcription after 24 h or of TCF/LEF-dependent transcription after 16 h following exposure to 1 μM BIO. hCMEC/D3 cells were transfected with pMDR-Luc and pCMV-SEAP or with Topflash DNA and pCMV-SEAP together with empty vector or dnTCF, treated the next day with BIO and later harvested for luciferase and alkaline phosphatase activity. Values shown are the fold increases in luciferase/alkaline phosphatase activity ratio over control untreated cells and are given as mean ± SEM. Number of experiments and statistical significance (paired _t_-test on the log of the ratios) are shown above each bar. (d) Western blots of brain endothelial cell lysates from rat (RBEC) or human (hCMEC/D3) cells either untreated or exposed to BIO at the concentrations shown for 5 days (rat) or for 24 h (human). The blots were probed with anti-p-gp monoclonal antibody C219. Equal protein loading was verified by probing with anti-β-actin antibody (shown) and Ponceau staining (not shown). Images shown are representative of three independent experiments performed.
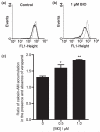
Fig. 3
Effect of GSK-3β inhibitor, BIO, on p-gp efflux activity in human immortalized brain endothelial cells. (a and b) Show fluorescence histograms of cells (a) untreated or (b) pre-treated with 1 μM BIO for 24 h and exposed either to vehicle for 30 min followed by 0.5 μM calcein-AM for a further 30 min (solid line) or the same procedure but with 20 μM verapamil present throughout (dotted line). Note in the presence of verapamil i.e. with p-gp activity blocked, accumulation of fluorescent calcein, as seen by the median values of the dotted line histograms, is similar in (a) and (b). In the absence of verapamil i.e. with p-gp activity still present, accumulation is significantly less in the BIO-treated cells as seen by comparing the median values of the solid-line histograms in (a) and (b). Median values of background fluorescence (histograms not shown) were very low i.e. 1.4 ± 0.4. (c) Show the increases in accumulation of calcein in the presence of 20 μM verapamil in untreated cells and in cells exposed to 0.5 or 1 μM BIO. Values shown are the mean ± SEM of results from four separate experiments and indicate significant differences from untreated controls for cells treated with 0.5 μM BIO (*p < 0.05) and with 1 μM BIO (**p < 0.01). Median values from fluorescence histograms as shown in (a and b) were used to derive these data.
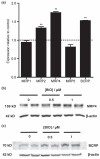
Fig. 4
Effect of GSK-3β inhibitor BIO on expression of other ABC transporters in human immortalized brain endothelial cells. Cells were exposed to 1 μM BIO for 16 h and then analysed (a) by qRT-PCR for expression of MRP1, MRP2, MRP4, MRP5 and BCRP. As described in legend to Fig. 2, expression was firstly estimated relative to reference genes. Values shown are the mean ± SEM of these levels of expression relative to that of control untreated cells. Significant increases over control are shown for MRP2 (**p < 0.01, n = 4), MRP4 (**p < 0.01, n = 4) and BCRP (**p < 0.01, n = 4) but not for MRP1 or MRP5. (b and c) Western blots showing expression of (b) MRP4 and (c) BCRP 24 h after exposure to BIO at the concentrations shown. Equal protein loading was verified by probing with anti-β-actin antibody (shown) and Ponceau staining (not shown). Images shown are representative of those obtained from three independent experiments.
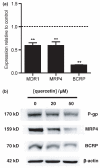
Fig. 5
Effect of quercetin on expression of ABC transporters, p-gp, MRP4 and BCRP in human immortalized brain endothelial cells. Cells were harvested 8 h following exposure to 50 μM quercetin and analysed at transcript level (a) for expression of MDR1, MRP4 and BCRP (**p < 0.01, n = 4 in each case). (b) Western blot analysis of lysates from cells 72 h following exposure to quercetin at the concentrations shown demonstrating decreases at the protein level in expression of p-gp, MRP4 and BCRP. The blots were probed sequentially with anti-p-gp monoclonal antibody C219, anti-MRP4 antibody, M41-10 and anti-BCRP antibody, BXP-21. Equal protein loading was verified by probing with anti-β-actin antibody (shown) and Ponceau staining (not shown). Images shown are representative of three independent experiments performed.
Fig. 6
Effect of Dkk-1 on expression of p-gp in rat brain endothelial cells (RBEC) and human immortalized brain endothelial cells. (a) Western blots showing decreased expression of p-gp in RBEC and in human immortalized brain endothelial cells (hCMEC/D3) following treatment with 0.2 μg/mL Dkk-1 for 24 h. The blots were probed with anti-p-gp monoclonal antibody C219 and then with anti-β-actin antibody to confirm equal protein loading per well. Images shown are representative of three independent experiments performed. (b) Immunocytochemical staining for β-catenin in human brain endothelial cells to show reduction in the presence of 0.2 μg/mL Dkk-1 of the nuclear/perinuclear localization brought about by 16 h exposure to 20 μM pan Wnt agonist.
Similar articles
- The cross-talk between canonical and non-canonical Wnt-dependent pathways regulates P-glycoprotein expression in human blood-brain barrier cells.
Pinzón-Daza ML, Salaroglio IC, Kopecka J, Garzòn R, Couraud PO, Ghigo D, Riganti C. Pinzón-Daza ML, et al. J Cereb Blood Flow Metab. 2014 Aug;34(8):1258-69. doi: 10.1038/jcbfm.2014.100. Epub 2014 Jun 4. J Cereb Blood Flow Metab. 2014. PMID: 24896565 Free PMC article. - 1α,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells.
Durk MR, Chan GN, Campos CR, Peart JC, Chow EC, Lee E, Cannon RE, Bendayan R, Miller DS, Pang KS. Durk MR, et al. J Neurochem. 2012 Dec;123(6):944-53. doi: 10.1111/jnc.12041. Epub 2012 Nov 1. J Neurochem. 2012. PMID: 23035695 Free PMC article. - Beta amyloid effects on expression of multidrug efflux transporters in brain endothelial cells.
Kania KD, Wijesuriya HC, Hladky SB, Barrand MA. Kania KD, et al. Brain Res. 2011 Oct 18;1418:1-11. doi: 10.1016/j.brainres.2011.08.044. Epub 2011 Aug 23. Brain Res. 2011. PMID: 21920506
Cited by
- Efflux transporters in blood-brain interfaces of the developing brain.
Strazielle N, Ghersi-Egea JF. Strazielle N, et al. Front Neurosci. 2015 Feb 5;9:21. doi: 10.3389/fnins.2015.00021. eCollection 2015. Front Neurosci. 2015. PMID: 25698917 Free PMC article. Review. - Methylation of SFRP5 is related to multidrug resistance in leukemia cells.
Wang H, Wang X, Hu R, Yang W, Liao A, Zhao C, Zhang J, Liu Z. Wang H, et al. Cancer Gene Ther. 2014 Feb;21(2):83-9. doi: 10.1038/cgt.2013.87. Epub 2014 Jan 17. Cancer Gene Ther. 2014. PMID: 24434572 - BATF2 reverses multidrug resistance of human gastric cancer cells by suppressing Wnt/β-catenin signaling.
Yang W, Wu B, Ma N, Wang Y, Guo J, Zhu J, Zhao S. Yang W, et al. In Vitro Cell Dev Biol Anim. 2019 Jun;55(6):445-452. doi: 10.1007/s11626-019-00360-5. Epub 2019 May 28. In Vitro Cell Dev Biol Anim. 2019. PMID: 31140101 - Overcoming multidrug resistance in human cancer cells by natural compounds.
Nabekura T. Nabekura T. Toxins (Basel). 2010 Jun;2(6):1207-24. doi: 10.3390/toxins2061207. Epub 2010 May 28. Toxins (Basel). 2010. PMID: 22069634 Free PMC article. Review. - Gsk3 Signalling and Redox Status in Bipolar Disorder: Evidence from Lithium Efficacy.
Luca A, Calandra C, Luca M. Luca A, et al. Oxid Med Cell Longev. 2016;2016:3030547. doi: 10.1155/2016/3030547. Epub 2016 Aug 18. Oxid Med Cell Longev. 2016. PMID: 27630757 Free PMC article. Review.
References
- Afonso PV, Ozden S, Prevost M-C, Schmitt C, Seilhean D, Weksler B, Couraud P-O, Gessain A, Romero IA, Ceccaldi PE. Human blood–brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganisation. J. Immunol. 2007;179:2576–2583. - PubMed
- Bailey-Dell KJ, Hassel B, Doyle LA, Ross DD. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim. Biophys. Acta. 2001;1520:234–241. - PubMed
- Cucullo L, Couraud P-O, Weklser B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J. Cereb. Blood Flow Metab. 2007;28:312–328. - PubMed
- Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev. Dyn. 2006;235:3110–3120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous