LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies - PubMed (original) (raw)
Review
LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies
Anna P Lillis et al. Physiol Rev. 2008 Jul.
Abstract
The LDL receptor-related protein (originally called LRP, but now referred to as LRP1) is a large endocytic receptor that is widely expressed in several tissues. LRP1 is a member of the LDL receptor family that plays diverse roles in various biological processes including lipoprotein metabolism, degradation of proteases, activation of lysosomal enzymes, and cellular entry of bacterial toxins and viruses. Deletion of the LRP1 gene leads to lethality in mice, revealing a critical, but as of yet, undefined role in development. Tissue-specific gene deletion studies reveal an important contribution of LRP1 in the vasculature, central nervous system, macrophages, and adipocytes. Three important properties of LRP1 dictate its diverse role in physiology: 1) its ability to recognize more than 30 distinct ligands, 2) its ability to bind a large number of cytoplasmic adaptor proteins via determinants located on its cytoplasmic domain in a phosphorylation-specific manner, and 3) its ability to associate with and modulate the activity of other transmembrane receptors such as integrins and receptor tyrosine kinases.
Figures
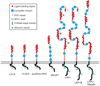
Figure 1. Modular domain organization of LDL receptor family members
In LRP1, the four clusters of complement-type repeats are numbered I – IV.
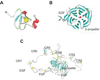
Figure 2. Structure of modules from LDL receptor family members
A. X-ray structure of CR7 from LRP1 (248) showing the basic folding of these modules with the structural calcium residue. B. X-ray structure of EGF and β-propeller (YWTD) domain from the LDL receptor (105) showing the six-bladed β-propeller domain. C. X-ray structure of the LDL receptor ectodomain solved at pH 5.2 (236) showing the interaction of CR4 and CR5 with the β-propeller domain at this reduced pH.
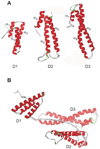
Figure 3. A. NMR structure of RAP domains 1 (D1) (298), D2 (130), and D3 (131) showing the three helical bundle organization of each domain
The helices are numbered as α1 – α9. B. Since the three domains of RAP are independent and do not interact, but are connected by long flexible loops, the protein is expected to adopt a variety of conformations in solution, one of which is shown.
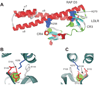
Figure 4. Structure of the RAP D3 in complex with two CR from the LDL receptor (68)
A. Lysines 256 and 270 are located in helix α8 of the D3 domain and provide the primary contacts with CR4 and CR3 of the LDLR, respectively. B and C. Detailed structure shows the acidic pocket surrounding K256 (B) and K270 (C). The structural calcium ion is shown. W144 and F105 are close to the aliphatic portion of the lysine residues in the pocket.
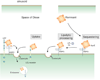
Figure 5. Proposed model for the involvement of LRP1 in remnant metabolism in the liver
The model is adapted from (145,148). Remnant lipoprotein particles entering the space of Disse in the liver are first thought to be sequestered by association with heparan sulfate proteoglycans (HSPG). Here they are remodeled by the action of lipoprotein lipase (LPL) and hepatic lipase (HL). Internalization by the hepatocytes is mediated directly by HSPG, the LDL receptor, or HSPG/LRP1 complexes.
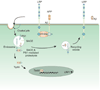
Figure 6. Proposed model of LRP1 involvement in the trafficking of APP and Aβ production
Fe65 bridges LRP1 and APP via cytoplasmic domain interactions, resulting in enhanced delivery of APP into endosomal compartments where BACE and PS1 are known to reside. Here, regulated intramembrane proteolysis of APP occurs, generating the Aβ peptide and releasing its intracellular domain. The APP intracellular domain forms a multimeric complex with Tip60 and Fe65, diffuses to the nucleus and modulates gene expression, including suppression of LRP1 gene transcription. The Aβ peptide is released into the media in recycling vesicles.
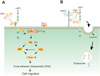
Figure 7. Role of LRP1 in mediating TSP1 signaling and metabolism
A. The hep I sequence (aa 17–35) of the N-terminal domain of TSP1 binds cell surface CRT (aa 19–36). When bound to TSP1 or the hep I peptide, CRT binding to LRP1 is enhanced and signaling through the CRT-LRP1 co-receptor complex is initiated. TSP1 binding to the CRT-LRP1 complex induces association of the Gαi2 protein subunit with LRP1. Phosphorylation of Src and FAK occurs downstream and leads to ERK and PI3K activation. This signaling cascade triggers inactivation of RhoA, resulting in focal adhesion disassembly (FAD) and stimulation of cell migration. In addition, TSP1 signaling through the hep I sequence requires the participation of Thy-1, a GPI-linked protein, to affect Src activation, although it does not appear that Thy-1 directly binds to either LRP1 or CRT(5) B. LRP1 mediates endocytosis of TSP1 through binding of the N-terminal domain of TSP1, a process which requires heparan sulfate proteoglycans (HSPG) for internalization (163,164,179,180).
Similar articles
- Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability.
Lillis AP, Mikhailenko I, Strickland DK. Lillis AP, et al. J Thromb Haemost. 2005 Aug;3(8):1884-93. doi: 10.1111/j.1538-7836.2005.01371.x. J Thromb Haemost. 2005. PMID: 16102056 Review. - Serpin-Enzyme Receptors LDL Receptor-Related Protein 1.
Strickland DK, Muratoglu SC, Antalis TM. Strickland DK, et al. Methods Enzymol. 2011;499:17-31. doi: 10.1016/B978-0-12-386471-0.00002-X. Methods Enzymol. 2011. PMID: 21683247 Free PMC article. - Low-density lipoprotein receptor-related protein-1: role in the regulation of vascular integrity.
Strickland DK, Au DT, Cunfer P, Muratoglu SC. Strickland DK, et al. Arterioscler Thromb Vasc Biol. 2014 Mar;34(3):487-98. doi: 10.1161/ATVBAHA.113.301924. Epub 2014 Feb 6. Arterioscler Thromb Vasc Biol. 2014. PMID: 24504736 Free PMC article. Review. - Diverse role of LDL receptor-related protein in the clearance of proteases and in signaling.
Strickland DK, Ranganathan S. Strickland DK, et al. J Thromb Haemost. 2003 Jul;1(7):1663-70. doi: 10.1046/j.1538-7836.2003.00330.x. J Thromb Haemost. 2003. PMID: 12871303 Review.
Cited by
- Microglia Ontology and Signaling.
ElAli A, Rivest S. ElAli A, et al. Front Cell Dev Biol. 2016 Jun 29;4:72. doi: 10.3389/fcell.2016.00072. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27446922 Free PMC article. Review. - Pharmacological Targeting of Plasminogen Activator Inhibitor-1 Decreases Vascular Smooth Muscle Cell Migration and Neointima Formation.
Ji Y, Weng Z, Fish P, Goyal N, Luo M, Myears SP, Strawn TL, Chandrasekar B, Wu J, Fay WP. Ji Y, et al. Arterioscler Thromb Vasc Biol. 2016 Nov;36(11):2167-2175. doi: 10.1161/ATVBAHA.116.308344. Epub 2016 Sep 22. Arterioscler Thromb Vasc Biol. 2016. PMID: 27659097 Free PMC article. - Impaired vascular-mediated clearance of brain amyloid beta in Alzheimer's disease: the role, regulation and restoration of LRP1.
Ramanathan A, Nelson AR, Sagare AP, Zlokovic BV. Ramanathan A, et al. Front Aging Neurosci. 2015 Jul 15;7:136. doi: 10.3389/fnagi.2015.00136. eCollection 2015. Front Aging Neurosci. 2015. PMID: 26236233 Free PMC article. Review. - Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis.
Cafaro A, Schietroma I, Sernicola L, Belli R, Campagna M, Mancini F, Farcomeni S, Pavone-Cossut MR, Borsetti A, Monini P, Ensoli B. Cafaro A, et al. Int J Mol Sci. 2024 Jan 30;25(3):1704. doi: 10.3390/ijms25031704. Int J Mol Sci. 2024. PMID: 38338977 Free PMC article. Review. - LRP1-dependent endocytic mechanism governs the signaling output of the bmp system in endothelial cells and in angiogenesis.
Pi X, Schmitt CE, Xie L, Portbury AL, Wu Y, Lockyer P, Dyer LA, Moser M, Bu G, Flynn EJ 3rd, Jin SW, Patterson C. Pi X, et al. Circ Res. 2012 Aug 17;111(5):564-74. doi: 10.1161/CIRCRESAHA.112.274597. Epub 2012 Jul 9. Circ Res. 2012. PMID: 22777006 Free PMC article.
References
- Abe Ji, Deguchi J, Matsumoto T, Takuwa N, Noda M, Ohno M, Makuuchi M, Kurokawa K, Takuwa Y. Stimulated Activation of Platelet-Derived Growth Factor Receptor In Vivo in Balloon-Injured Arteries : A Link Between Angiotensin II and Intimal Thickening. Circulation. 1997;96:1906–1913. - PubMed
- Argraves KM, Battey FD, MacCalman CD, McCrae KR, Gåfvels M, Kozarsky KF, Chappell DA, Strauss JF, Strickland DK. The very low density lipoprotein receptor mediates the cellular catabolism of lipoprotein lipase and urokinase-plasminogen activator inhibitor type I complexes. J Biol Chem. 1995;270:26550–26557. - PubMed
- Baker RN, Cancilla PA, Pollock PS, Frommes SP. The movement of exogenous protein in experimental cerebral edema. An electron microscopic study after freeze-injury. J Neuropathol Exp Neurol. 1971;30:668–679. - PubMed
- Barker TH, Pallero MA, MacEwen MW, Tilden SG, Woods A, Murphy-Ullrich JE, Hagood JS. Thrombospondin-1-induced focal adhesion disassembly in fibroblasts requires Thy-1 surface expression, lipid raft integrity, and Src activation. J Biol Chem. 2004;279:23510–23516. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL050784/HL/NHLBI NIH HHS/United States
- T32 HL007698/HL/NHLBI NIH HHS/United States
- P01 HL054710/HL/NHLBI NIH HHS/United States
- R01 HL072929/HL/NHLBI NIH HHS/United States
- T32 GM 07171/GM/NIGMS NIH HHS/United States
- T32 GM 08361-16/GM/NIGMS NIH HHS/United States
- HL 72929/HL/NHLBI NIH HHS/United States
- T32 HL 07698/HL/NHLBI NIH HHS/United States
- R01 HL079644/HL/NHLBI NIH HHS/United States
- HL 79644/HL/NHLBI NIH HHS/United States
- T32 GM007171/GM/NIGMS NIH HHS/United States
- HL 54710/HL/NHLBI NIH HHS/United States
- T32 GM008361/GM/NIGMS NIH HHS/United States
- HL 50784/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous