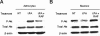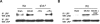Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain - PubMed (original) (raw)
Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein induce Akt phosphorylation in the ischemic brain
Jie An et al. Blood. 2008.
Abstract
Tissue-type plasminogen activator (tPA) is found in the intravascular space and in the central nervous system. The low-density lipoprotein receptor-related protein (LRP) is expressed in neurons and in perivascular astrocytes. During cerebral ischemia, tPA induces the shedding of LRP's extracellular domain from perivascular astrocytes, and this is followed by the development of cerebral edema. Protein kinase B (Akt) is a serine/threonine kinase that plays a critical role not only in cell survival but also in the regulation of the permeability of the blood-brain barrier. We found that, in the early phases of the ischemic insult, the interaction between tPA and LRP induces Akt phosphorylation (pAkt) in perivascular astrocytes and inhibits pAkt in neurons. Coimmunoprecipitation studies indicate that pAkt and LRP's intracellular domain interact in perivascular astrocytes and that this interaction is dependent on the presence of tPA and results in the development of edema. Together, these results indicate that, in the early stages of cerebral ischemia, the interaction between tPA and LRP in perivascular astrocytes induces the activation of a cell signaling event mediated by pAkt that leads to increase in the permeability of the blood-brain barrier.
Figures
Figure 1
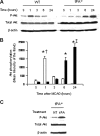
Effect of tPA deficiency on Akt phosphorylation in the ischemic brain. (A) Representative Western blot analysis for Akt phosphorylated at serine 473 (pAkt) and total Akt in brain extracts from wild-type (WT) and tPA-deficient (tPA−/−) mice 1 to 24 hours after middle cerebral artery occlusion (MCAO). S denotes sham-operated mice. (B) Mean density of the band of 6 immunoblots for pAkt in wild-type (□) and tPA−/− mice (■) 1 to 24 hours after MCAO. n = 6. Lines depict SD. *P < .001 relative to either WT or tPA−/− sham-operated mice. †P < .001 compared with tPA−/− mice 1 hour after MCAO. ‡P < .001 relative to WT mice 24 hours after MCAO. (C) Representative Western blot analysis for pAkt in brain extracts from tPA−/− mice 1 hour after middle cerebral artery occlusion (MCAO) and either no treatment (NT) or the intracerebral injection of tPA (tPA).
Figure 2
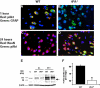
Effect of tPA deficiency on ischemic cell death. (A-D) Immunohistochemical analysis of neuronal pAkt in the area of interest-2 (AOI-2) 1 (A,B) and 24 (C,D) hours after middle cerebral artery occlusion (MCAO) in wild-type (A,C) and tPA−/− (B,D) mice. Blue indicates 4′,6-diamidino-2-phenylindole and red indicates pAkt in panels A and B and NeuN in panels C and D. Green indicates GFAP in panels A and B and pAkt in panels C and D. Original magnification, A-D, 40×. Images were visualized using a Leica DMRBE microscope (Leica, Houston, TX) equipped with a 100×/1.30 numeric aperture (NA) and a LeicaDC500 camera. Images were processed using software provided by the camera manufacturer. (E) Representative Western blot analysis of PARP-1 cleavage in brain extracts from wild-type (WT) and tPA−/− mice 6 and 24 hours after MCAO. C denotes a control WT animal. The arrow indicates an approximately 89-kDa PARP-1 cleavage product. Each observation was repeated 4 times. (F) Mean percentage of TUNEL-positive cells in the area of ischemic penumbra in WT and tPA−/− mice 24 hours after MCAO; n = 7. *P < .005 compared with WT mice.
Figure 3
tPA induces Akt phosphorylation in perivascular astrocytes. (A) Representative micrographs of immunogold electron microscopy analysis for pAkt in perivascular astrocytes in the area of interest-2 (AOI-2) of wild-type (i) and tPA−/− mice (ii) 1 hour after MCAO. Bv indicates blood vessel; and bm, basement membrane. Each observation was repeated 4 times. (B) Mean Evans blue dye extravasation in wild-type mice 6 hours after reperfusion and the intraventricular injection of either vehicle (control, ■) or wortmannin (□). Results are given as a percentage compared with control-treated mice. Lines depict SD; n = 8. *P < .005 compared with control-treated mice.
Figure 4
tPA induces Akt phosphorylation via a plasminogen-independent mechanism. (A,B) Representative Western blot analysis of pAkt and total Akt in brain extracts from Plg−/− mice (A) and tPA−/− animals (B) 1 hour after MCAO. A subset of tPA−/− mice was either left untreated (NT) or injected directly into the ischemic tissue with murine tPA (tPA) or a combination of tPA and RAP (tPA + RAP). S denotes sham-operated animal. Each observation was repeated 3 times.
Figure 5
The effect of the interaction between tPA and LRP on pAkt is cell type– specific. (A,B) Representative Western blot analysis of pAkt and total Akt in extracts from astrocytic (A) and neuronal (B) cultures after 1 hour of exposure to oxygen-glucose deprivation (OGD) conditions and incubation with either tPA or a combination of tPA and the receptor-associated protein (RAP). RAP is a molecular chaperone that inhibits the binding of LRP to its ligands. NT indicates no treatment. Each observation was repeated 4 times.
Figure 6
LRP's intracellular domain and pAkt interact in perivascular astrocytes. (A) Coimmunoprecipitation studies in brain extracts from Wt and tPA−/− mice 1 hour after MCAO. tPA−/− were either left untreated (−tPA) or injected directly into the ischemic tissue with tPA (1 μM;+tPA). (B) Coimmunoprecipitation studies in brain extracts from Wt mice 1 hour after MCAO. Animals were either left untreated (MCAO) or injected into the ischemic tissue with RAP (9 μM; MCAO + RAP).
Similar articles
- Tissue-type plasminogen activator-mediated shedding of astrocytic low-density lipoprotein receptor-related protein increases the permeability of the neurovascular unit.
Polavarapu R, Gongora MC, Yi H, Ranganthan S, Lawrence DA, Strickland D, Yepes M. Polavarapu R, et al. Blood. 2007 Apr 15;109(8):3270-8. doi: 10.1182/blood-2006-08-043125. Epub 2006 Dec 14. Blood. 2007. PMID: 17170123 Free PMC article. - Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation.
Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Zhang X, et al. Am J Pathol. 2007 Oct;171(4):1281-90. doi: 10.2353/ajpath.2007.070472. Epub 2007 Aug 23. Am J Pathol. 2007. PMID: 17717150 Free PMC article. - Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein.
Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Yepes M, et al. J Clin Invest. 2003 Nov;112(10):1533-40. doi: 10.1172/JCI19212. J Clin Invest. 2003. PMID: 14617754 Free PMC article. - Reprint of: Fibrinolytic and Non-fibrinolytic Roles of Tissue-type Plasminogen Activator in the Ischemic Brain.
Yepes M. Yepes M. Neuroscience. 2024 Jul 9;550:21-29. doi: 10.1016/j.neuroscience.2024.05.040. Epub 2024 Jul 2. Neuroscience. 2024. PMID: 38964373 Review. - Fibrinolytic and Non-fibrinolytic Roles of Tissue-type Plasminogen Activator in the Ischemic Brain.
Yepes M. Yepes M. Neuroscience. 2024 Mar 26;542:69-80. doi: 10.1016/j.neuroscience.2023.08.011. Epub 2023 Aug 11. Neuroscience. 2024. PMID: 37574107 Review.
Cited by
- Tumor necrosis factor-like weak inducer of apoptosis and fibroblast growth factor-inducible 14 mediate cerebral ischemia-induced poly(ADP-ribose) polymerase-1 activation and neuronal death.
Haile WB, Echeverry R, Wu F, Guzman J, An J, Wu J, Yepes M. Haile WB, et al. Neuroscience. 2010 Dec 29;171(4):1256-64. doi: 10.1016/j.neuroscience.2010.10.029. Epub 2010 Oct 16. Neuroscience. 2010. PMID: 20955770 Free PMC article. - Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis.
Gaultier A, Wu X, Le Moan N, Takimoto S, Mukandala G, Akassoglou K, Campana WM, Gonias SL. Gaultier A, et al. J Cell Sci. 2009 Apr 15;122(Pt 8):1155-62. doi: 10.1242/jcs.040717. Epub 2009 Mar 19. J Cell Sci. 2009. PMID: 19299462 Free PMC article. - Pharmacological Inhibition of HDAC6 Attenuates NLRP3 Inflammatory Response and Protects Dopaminergic Neurons in Experimental Models of Parkinson's Disease.
Yan S, Wei X, Jian W, Qin Y, Liu J, Zhu S, Jiang F, Lou H, Zhang B. Yan S, et al. Front Aging Neurosci. 2020 Mar 31;12:78. doi: 10.3389/fnagi.2020.00078. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32296327 Free PMC article. - Hydrogen Sulfide Attenuates Tissue Plasminogen Activator-Induced Cerebral Hemorrhage Following Experimental Stroke.
Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J. Liu H, et al. Transl Stroke Res. 2016 Jun;7(3):209-19. doi: 10.1007/s12975-016-0459-5. Epub 2016 Mar 28. Transl Stroke Res. 2016. PMID: 27018013 - One-Compound-Multi-Target: Combination Prospect of Natural Compounds with Thrombolytic Therapy in Acute Ischemic Stroke.
Chen HS, Qi SH, Shen JG. Chen HS, et al. Curr Neuropharmacol. 2017;15(1):134-156. doi: 10.2174/1570159x14666160620102055. Curr Neuropharmacol. 2017. PMID: 27334020 Free PMC article. Review.
References
- National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. - PubMed
- Wang YF, Tsirka SE, Strickland S, et al. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. - PubMed
- Yepes M, Sandkvist M, Wong MK, et al. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous