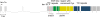Caenorhabditis elegans teneurin, ten-1, is required for gonadal and pharyngeal basement membrane integrity and acts redundantly with integrin ina-1 and dystroglycan dgn-1 - PubMed (original) (raw)
Caenorhabditis elegans teneurin, ten-1, is required for gonadal and pharyngeal basement membrane integrity and acts redundantly with integrin ina-1 and dystroglycan dgn-1
Agnieszka Trzebiatowska et al. Mol Biol Cell. 2008 Sep.
Abstract
The Caenorhabditis elegans teneurin ortholog, ten-1, plays an important role in gonad and pharynx development. We found that lack of TEN-1 does not affect germline proliferation but leads to local basement membrane deficiency and early gonad disruption. Teneurin is expressed in the somatic precursor cells of the gonad that appear to be crucial for gonad epithelialization and basement membrane integrity. Ten-1 null mutants also arrest as L1 larvae with malformed pharynges and disorganized pharyngeal basement membranes. The pleiotropic phenotype of ten-1 mutant worms is similar to defects found in basement membrane receptor mutants ina-1 and dgn-1 as well as in the mutants of the extracellular matrix component laminin, epi-1. We show that the ten-1 mutation is synthetic lethal with mutations of genes encoding basement membrane components and receptors due to pharyngeal or hypodermal defects. This indicates that TEN-1 could act redundantly with integrin INA-1, dystroglycan DGN-1, and laminin EPI-1 in C. elegans development. Moreover, ten-1 deletion sensitizes worms to loss of nidogen nid-1 causing a pharynx unattached phenotype in ten-1;nid-1 double mutants. We conclude that TEN-1 is important for basement membrane maintenance and/or adhesion in particular organs and affects the function of somatic gonad precursor cells.
Figures
Figure 1.
Genomic organization of ten-1 gene and location of tm651 and ok641 deletions. Exons are depicted as boxes and introns are shown as lines. Expression of ten-1 is regulated by alternative promoters: ten-1a and ten-1b, resulting in two type II transmembrane protein variants differing in the length of their intracellular domain. Fragments of exons encoding different protein domains are labeled as follows: red, single transmembrane domain, green, EGF-like repeats in two groups, yellow, region of conserved cysteines, and blue, stretch of YD repeats. Black horizontal lines show the regions deleted in two ten-1 mutants: tm651 and ok641.
Figure 2.
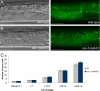
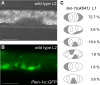
Germ cells are released from the early gonad of ten-1(ok641) mutant through the central break. Germ cell number and localization were evaluated using the P-granule marker pie-1::GFP::PGL-1. (A) Wild-type L3 gonad. The somatic gonadal primordium forms in the middle of the gonad, and germ cells fill the two gonad arms (only one arm is shown). (B) Ruptured gonadal primordium of a ten-1(ok641) L3 larva. Germ cells are released into the body cavity and localize in the vicinity of the developing somatic gonad primordium. (C) Time course of germline development in wild-type animals and ten-1(ok641) mutants. There is no germline overproliferation in the early gonads of the ten-1(ok641) mutant. Scale bar, 20 μmm.
Figure 3.
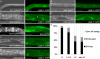
The basement membrane breaks on the dorsal side of the ten-1(ok641) gonads. Basement membranes were visualized by the LAM-1::GFP marker (A–D and F–G) and the anti-LET-2 immunostaining (E). The ten-1(ok641) L1 gonad (A), wild-type L1 (B), and L2 (D) gonads are uniformly covered by laminin. (C) In the ten-1(ok641) mutant, the gonadal basement membrane becomes thinner or fails to assemble correctly (arrowhead) at the L2 stage. (E) Lack of gonadal BM on the dorsal side of ten-1(ok641) L2 gonad is visualized by collagen IV immunostaining with anti-LET-2. (F) There is no laminin present in the center of the ten-1(ok641) L3 gonad. The basement membrane is absent completely, and germ cells are released. (G) The wild-type L3 gonad is entirely covered by laminin. (H) Time-course analysis of gonadal BM integrity in ten-1(ok641) worms carrying the LAM-1::GFP marker. Scale bar, 20 μmm.
Figure 4.
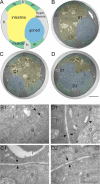
Basement membrane ultrastructure in ten-1 mutant worms. Schematic cross-section through the midbody of wild-type worm (A). Transmission electron microscopy sections of a wild-type L3 (B) and a ten-1(ok641) mutant (C and D). Tissues are labeled as follows: blue, gonad; yellow, intestine; green, muscles; and gray/unlabeled, hypodermis. Enlargements (B1–D2) are marked on the cross-sections (B–D) with white rectangles. Morphology of wild-type BMs at the boundaries between gonad and intestine (B1, arrows). The gonadal and intestinal BM of the ten-1 mutant appears wild-type in a section 2 μmm distant from the central break (C1, arrows). In the midbody region, the mutant gonad breaks on its dorsal side, and there is no BM present between germ cells and intestine (D1, arrowhead). However, BMs between intestine and hypodermis (D1, arrows) or ventral gonad and hypodermal ridge (D2, arrows) have a normal ultrastructure. Scale bar, 5 μmm (A–D) and 500 nm (B1–D2).
Figure 5.
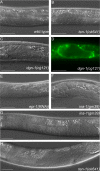
Misshapen gonadal primordia are found in several basement membrane mutants, i.e., dystroglycan dgn-1, integrin ina-1, and laminin epi-1(RNAi) worms. DIC pictures of early gonads in wild type (A), ten-1(ok641) (B), dgn-1(cg121) (C), and the corresponding LAM-1::GFP pattern (D), epi-1(RNAi) (E), ina-1(gm39) L2 larva (F), ina-1(gm39) L4 larva (G), and ten-1(ok641) L4 larva (H). Mutant gonads do not form a tube-like structure but grow into a disorganized mass. Scale bar, 20 μmm.
Figure 6.
Teneurin is expressed in somatic cells of the early gonads and SGPs are mislocalized in the L1 gonads of ten-1 mutants. Expression from the upstream promoter of ten-1 is found in the SGPs of L2 gonads in wild-type worms (A and B). In C we present a schematic representation of the position of the Z1 and Z4 (gray shading) and Z2 and Z3 (white) cells in gonads of ten-1(ok641) L1 larvae carrying the lag-2::gfp marker (n = 55). We found that Z1 and Z4 cells are often mispositioned, and the percentage of animals showing the observed patterns is indicated to the right. Scale bar, 20 μmm.
Figure 7.
Morphological defects found in epi-1(RNAi) worms, ten-1(ok641); epi-1(RNAi) animals, and ten-1; ina-1 double mutants. Wild-type (A) and ten-1(ok641) L1 larvae (B). _epi-1_-depleted worms are often misshapen, but defects are relatively mild (C). Arrested larva of ina-1(gm144) mutant (D). Morphological defects of epi-1(RNAi) worms were enhanced by ten-1(ok641) deletion and caused deformation of the entire body in the arrested larvae (E). Similar defects were found in ten-1(ok641);ina-1(gm144) double mutants (F). Severity and penetrance of the defects were greatly enhanced in the double mutants compared with single mutants. Scale bar, 20 μmm.
Figure 8.
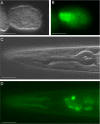
The long TEN-1 isoform is expressed in the developing and adult pharynx. The GFP::TEN-1 transgene (kdEx121) is expressed in the developing pharynx of the early embryo (A and B) and outlines the adult pharynx (C and D). Expression of the kdEx121 is also found in some head neurons (D). Scale bar, 20 μm.
Figure 9.
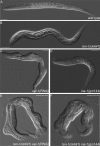
Pharyngeal defects in ten-1, nid-1, and dgn-1 single and double mutants. Pharynx morphology of L1 larva is shown. LAM-1::GFP marker labels the pharyngeal basement membrane. Wild-type pharynx is outlined by a sharp DIC boundary visible by DIC microscopy (A). Basement membrane organization in the wild-type larva visualized by LAM-1::GFP (B). Arrested larvae of ten-1(ok641) mutant have misshapen pharynges and the pharyngeal outline is invisible on DIC pictures (C). In the ten-1 mutant, the basement membrane around the pharynx is disorganized or missing in some parts (arrows) (D). The pharynx of the dgn-1 mutant worms shows no obvious defects (E). Arrested larvae of nid-1 mutants have sometimes bent pharynges (arrow) (F) or their pharynges do not attach to the hypoderm (similar to the double mutant shown in H). Variably misshapen pharynges were found in the ten-1;dgn-1 double mutants (G). An unattached pharynx (Pun) phenotype observed in ten-1;nid-1 double mutants (H). White arrowheads mark the anterior and black arrowheads posterior ends of the pharynges. Scale bar, 20 μm.
Comment in
- Mol Biol Cell. 19:3615.
Similar articles
- C. elegans dystroglycan DGN-1 functions in epithelia and neurons, but not muscle, and independently of dystrophin.
Johnson RP, Kang SH, Kramer JM. Johnson RP, et al. Development. 2006 May;133(10):1911-21. doi: 10.1242/dev.02363. Epub 2006 Apr 12. Development. 2006. PMID: 16611689 - Organ Length Control by an ADAMTS Extracellular Protease in Caenorhabditis elegans.
Shibata Y, Kawakado Y, Hori N, Tanaka K, Inoue R, Takano T, Kubota Y, Nishiwaki K. Shibata Y, et al. G3 (Bethesda). 2016 May 3;6(5):1449-57. doi: 10.1534/g3.116.028019. G3 (Bethesda). 2016. PMID: 26994289 Free PMC article. - Basement membranes.
Kramer JM. Kramer JM. WormBook. 2005 Sep 1:1-15. doi: 10.1895/wormbook.1.16.1. WormBook. 2005. PMID: 18050423 Free PMC article. Review. - Ancient Function of Teneurins in Tissue Organization and Neuronal Guidance in the Nematode Caenorhabditis elegans.
Topf U, Drabikowski K. Topf U, et al. Front Neurosci. 2019 Mar 8;13:205. doi: 10.3389/fnins.2019.00205. eCollection 2019. Front Neurosci. 2019. PMID: 30906249 Free PMC article. Review.
Cited by
- C-terminal region of teneurin-1 co-localizes with the dystroglycan complex in adult mouse testes and regulates testicular size and testosterone production.
Chand D, Colacci M, Dixon K, Kollara A, Brown TJ, Lovejoy DA. Chand D, et al. Histochem Cell Biol. 2014 Feb;141(2):191-211. doi: 10.1007/s00418-013-1154-1. Epub 2013 Oct 24. Histochem Cell Biol. 2014. PMID: 24154551 - A Putative Role of Teneurin-2 and Its Related Proteins in Astrocytes.
Tessarin GWL, Michalec OM, Torres-da-Silva KR, Da Silva AV, Cruz-Rizzolo RJ, Gonçalves A, Gasparini DC, Horta-Júnior JAC, Ervolino E, Bittencourt JC, Lovejoy DA, Casatti CA. Tessarin GWL, et al. Front Neurosci. 2019 Jun 27;13:655. doi: 10.3389/fnins.2019.00655. eCollection 2019. Front Neurosci. 2019. PMID: 31316338 Free PMC article. - Evidence from ileum and liver transcriptomes of resistance to high-salt and water-deprivation conditions in camel.
Zhang D, Pan J, Zhou H, Cao Y. Zhang D, et al. Zoological Lett. 2020 Jun 5;6:8. doi: 10.1186/s40851-020-00159-3. eCollection 2020. Zoological Lett. 2020. PMID: 32518679 Free PMC article. - Ancient interaction between the teneurin C-terminal associated peptides (TCAP) and latrophilin ligand-receptor coupling: a role in behavior.
Woelfle R, D'Aquila AL, Pavlović T, Husić M, Lovejoy DA. Woelfle R, et al. Front Neurosci. 2015 Apr 24;9:146. doi: 10.3389/fnins.2015.00146. eCollection 2015. Front Neurosci. 2015. PMID: 25964737 Free PMC article. Review. - The ArfGEF GBF-1 Is Required for ER Structure, Secretion and Endocytic Transport in C. elegans.
Ackema KB, Sauder U, Solinger JA, Spang A. Ackema KB, et al. PLoS One. 2013 Jun 19;8(6):e67076. doi: 10.1371/journal.pone.0067076. Print 2013. PLoS One. 2013. PMID: 23840591 Free PMC article.
References
- Bagutti C., Forro G., Ferralli J., Rubin B., Chiquet-Ehrismann R. The intracellular domain of teneurin-2 has a nuclear function and represses zic-1-mediated transcription. J. Cell Sci. 2003;116:2957–2966. - PubMed
- Baum P. D., Garriga G. Neuronal migrations and axon fasciculation are disrupted in ina-1 integrin mutants. Neuron. 1997;19:51–62. - PubMed
- Baumgartner S., Chiquet-Ehrismann R. Tena, a Drosophila gene related to tenascin, shows selective transcript localization. Mech. Dev. 1993;40:165–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous