On the nature of partial agonism in the nicotinic receptor superfamily - PubMed (original) (raw)
On the nature of partial agonism in the nicotinic receptor superfamily
Remigijus Lape et al. Nature. 2008.
Abstract
Partial agonists are ligands that bind to receptors but produce only a small maximum response even at concentrations where all receptors are occupied. In the case of ligand-activated ion channels, it has been supposed since 1957 that partial agonists evoke a small response because they are inefficient at eliciting the change of conformation between shut and open states of the channel. We have investigated partial agonists for two members of the nicotinic superfamily-the muscle nicotinic acetylcholine receptor and the glycine receptor-and find that the open-shut reaction is similar for both full and partial agonists, but the response to partial agonists is limited by an earlier conformation change ('flipping') that takes place while the channel is still shut. This has implications for the interpretation of structural studies, and in the future, for the design of partial agonists for therapeutic use.
Figures
Figure 1
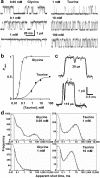
Glycine channels show short interruptions when activated by either full or partial agonists. a, Single-channel currents (opening upwards) produced by a full agonist (Glycine) or a partial agonist (Taurine). Open probability increases with agonist concentration (b) reaching a maximum of 0.54 for taurine and 0.96 for glycine (17 - 60 clusters, 3 - 4 patches per point; Hill equation fits; glycine data from ref. 2). c, Short shut times are clearly detectable in channel activations by 1 mM taurine. Points are the digitised record and the continuous line is the best time-course fit obtained during idealization together with the estimated duration of the short gaps. d, Short shut times are the predominant component in shut-time distributions for both agonists. With the usual resolution of 30 μs, a large proportion of such short shuttings are missed, but quite enough are observed to obtain a good estimate of their duration. Dwell-time distributions were fitted here and in Fig. 4d with a mixture of exponentials using
ekdist
, so they are descriptive and not mechanism dependent.
Figure 2
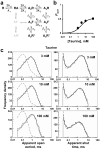
Global fit of the taurine data with a ‘flip’ mechanism provides a good description of the observations. a, The ‘flip’ activation mechanism fitted by Burzomato et al. (2004) to α1β channel activations by glycine. The success of the fit to the idealised records is judged by its ability to describe the experimental observations displayed in various ways, as in b and c. The results of the fit predict accurately the channel open probability (b) and apparent open- and shut-time distributions at all concentrations as shown by the continuous lines (fit predictions) superimposed onto the data points and the histograms. The dashed lines in the histograms are the distributions predicted if the records were obtained with perfect resolution (i.e. no events were missed; full data display Supplementary Figure 1 and Supplementary Table 1).
Figure 3
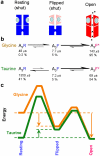
Activation of the glycine channel by the full agonist glycine and the partial agonist taurine. a, The three states of the fully-saturated receptor. The resting (shut) channel changes conformation to reach a partially-activated state (‘flipped’), still shut, but with increased affinity for the agonist (blue ellipses). It is from this flipped state that the channel can open (red, right). b, The reaction rates for channel activation by glycine and taurine differ largely in the first step, the flipping. Relative rates are indicated by the size of arrows. The diagram shows also the mean lifetime of each state (μs) and the proportion of time (percent) spent by a bound channel in each of the states at equilibrium. A channel occupied by glycine doesn’t stay long in the resting state, but quickly flips. When taurine is bound, the channel takes a long time to flip, so it spends much more time in the closed resting state. Once the channel has reached the flipped intermediate state, it opens quickly, regardless of which agonist is bound. The probability of opening, rather than unflipping, is over 96% for both agonists, so a burst of many openings results. c, An energy diagram for the three states of the saturated receptor, for full agonist (glycine, orange) and partial agonist (taurine, green). Again, the difference lies largely in the resting-flipped transition. As indicated by arrows, the transition from resting to flipped is downhill for glycine, but uphill for taurine. The overall transition from resting to open is very downhill for glycine, but almost level for taurine, as expected from the maximum response of about 54% open. The calculations used a frequency factor of 107 s−1 and the lines are shifted vertically so they meet at the open state., See also Supplementary movie.
Figure 4
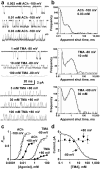
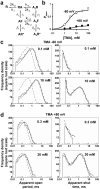
Short interruptions in openings of muscle nicotinic channels occur with both partial and full agonists and cannot be attributed entirely to channel block. a, Single channel currents produced by a full agonist (ACh) and a partial agonist (TMA, openings downwards in the first six rows). b, Short shut times are a major feature of shut-time distributions irrespective of agonist, voltage and concentration (see Supplementary Fig 2 and 4). Some of these brief shuttings could result from fast open-channel block at negative potentials, but not at positive potential, when open channel block is essentially absent. c, Open probability reaches a maximum of 0.94 (± 0.004) for ACh at −100 mV but is lower (0.59± 0.01) for ACh at +80 mV and for TMA at −80 or + 80 mV, 0.78 ± 0.05 or 0.16 ± 0.02, respectively (2 - 4 patches per point; Hill equation fits). These fits give also, for ACh −100 mV: _EC_50= 5.3± 0.2 μM; _n_H= 1.7± 0.1; for ACh +80 mV: _EC_50= 29± 2 μM; _n_H= 1.7 ± 0.1; for TMA −80 mV: _EC_50= 2.2 ± 0.5 mM; _n_H= 1.3 ± 0.4, and for TMA +80 mV: _EC_50= 6.4 ± 1.9 mM; _n_H= 1.2 ± 0.4. d, Because of open-channel block, the amplitude of single-channel currents declines with increasing TMA concentrations at negative potentials (open circles), but not at positive transmembrane potentials (filled circles, 2-3 patches per point).
Figure 5
The ‘flip’ mechanism describes well the activation of acetylcholine receptors by TMA. a, Schematic representation of the ‘flip’ mechanism used here: this has two agonist binding sites. As in Figure 2, the experimental data are displayed as channel open probability values (b) or dwell-time distributions (c, d). The results of fitting the idealised records are used to calculate the predicted _P_open - concentration curves and distributions (continuous lines). Both the open probability values and the dwell time distributions are well described by the fitted rate constants for TMA, at both −80 mV (c) and +80 mV (d). The dashed lines in the histograms are the distributions predicted if the records were obtained with perfect resolution (i.e. no events were missed). The full data display for the fits for ACh and TMA is in Supplementary Figures 2, 4, 5 and Supplementary Table 2).
Comment in
- Pharmacology: Unready for action.
Steinbach JH. Steinbach JH. Nature. 2008 Aug 7;454(7205):704-5. doi: 10.1038/454704a. Nature. 2008. PMID: 18685692 No abstract available.
Similar articles
- Pharmacology: Unready for action.
Steinbach JH. Steinbach JH. Nature. 2008 Aug 7;454(7205):704-5. doi: 10.1038/454704a. Nature. 2008. PMID: 18685692 No abstract available. - Disruption of a putative intersubunit electrostatic bond enhances agonist efficacy at the human α1 glycine receptor.
Welsh BT, Todorovic J, Kirson D, Allen HM, Bayly MD, Mihic SJ. Welsh BT, et al. Brain Res. 2017 Feb 15;1657:148-155. doi: 10.1016/j.brainres.2016.11.024. Epub 2016 Dec 5. Brain Res. 2017. PMID: 27923639 Free PMC article. - Functional probes of drug-receptor interactions implicated by structural studies: Cys-loop receptors provide a fertile testing ground.
Van Arnam EB, Dougherty DA. Van Arnam EB, et al. J Med Chem. 2014 Aug 14;57(15):6289-300. doi: 10.1021/jm500023m. Epub 2014 Mar 10. J Med Chem. 2014. PMID: 24568098 Free PMC article. Review. - What single-channel analysis tells us of the activation mechanism of ligand-gated channels: the case of the glycine receptor.
Sivilotti LG. Sivilotti LG. J Physiol. 2010 Jan 1;588(Pt 1):45-58. doi: 10.1113/jphysiol.2009.178525. Epub 2009 Sep 21. J Physiol. 2010. PMID: 19770192 Free PMC article. Review.
Cited by
- Perspectives on: conformational coupling in ion channels: allosteric coupling in ligand-gated ion channels.
Colquhoun D, Lape R. Colquhoun D, et al. J Gen Physiol. 2012 Dec;140(6):599-612. doi: 10.1085/jgp.201210844. J Gen Physiol. 2012. PMID: 23183696 Free PMC article. No abstract available. - Efficient maximum likelihood estimation of kinetic rate constants from macroscopic currents.
Stepanyuk AR, Borisyuk AL, Belan PV. Stepanyuk AR, et al. PLoS One. 2011;6(12):e29731. doi: 10.1371/journal.pone.0029731. Epub 2011 Dec 29. PLoS One. 2011. PMID: 22242142 Free PMC article. - Partial agonism of taurine at gamma-containing native and recombinant GABAA receptors.
Kletke O, Gisselmann G, May A, Hatt H, Sergeeva OA. Kletke O, et al. PLoS One. 2013 Apr 30;8(4):e61733. doi: 10.1371/journal.pone.0061733. Print 2013. PLoS One. 2013. PMID: 23637894 Free PMC article. - Ligand Binding at the 4-4 Agonist-Binding Site of the 42 nAChR Triggers Receptor Activation through a Pre-Activated Conformational State.
Indurthi DC, Lewis TM, Ahring PK, Balle T, Chebib M, Absalom NL. Indurthi DC, et al. PLoS One. 2016 Aug 23;11(8):e0161154. doi: 10.1371/journal.pone.0161154. eCollection 2016. PLoS One. 2016. PMID: 27552221 Free PMC article. - The α1K276E startle disease mutation reveals multiple intermediate states in the gating of glycine receptors.
Lape R, Plested AJ, Moroni M, Colquhoun D, Sivilotti LG. Lape R, et al. J Neurosci. 2012 Jan 25;32(4):1336-52. doi: 10.1523/JNEUROSCI.4346-11.2012. J Neurosci. 2012. PMID: 22279218 Free PMC article.
References
- del Castillo J, Katz B. Interaction at end-plate receptors between different choline derivatives. Proc. R. Soc. Lond. B. 1957;146:369–381. - PubMed
- Wyman J, Allen DW. The problem of the heme Interactions in hemoglobin and the basis of the Bohr effect. J. Polym. Sci. 1951;VII:499–518.
- Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources