Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination - PubMed (original) (raw)
Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination
Adan Aguirre et al. Neuron Glia Biol. 2007 Aug.
Abstract
Neural progenitor cells that express the NG2 proteoglycan are present in different regions of the adult mammalian brain where they display distinct morphologies and proliferative rates. In the developing postnatal and adult mouse, NG2(+) cells represent a major cell population of the subventricular zone (SVZ). NG2(+) cells divide in the anterior and lateral region of the SVZ, and are stimulated to proliferate and migrate out of the SVZ by focal demyelination of the corpus callosum (CC). Many NG2(+) cells are labeled by GFP-retrovirus injection into the adult SVZ, demonstrating that NG2(+) cells actively proliferate under physiological conditions and after demyelination. Under normal physiological conditions and after focal demyelination, proliferation of NG2(+) cells is significantly attenuated in wa2 mice, which are characterized by reduced signaling of the epidermal growth factor receptor (EGFR). This results in reduced SVZ-to-lesion migration of NG2(+) cells and oligodendrogenesis in the lesion. Expression of vascular endothelial growth factor (VEGF) and EGFR ligands, such as heparin binding-EGF and transforming growth factor alpha, is upregulated in the SVZ after focal demyelination of the CC. EGF-induced oligodendrogenesis and myelin protein expression in wild-type SVZ cells in culture are significantly attenuated in wa2 SVZ cells. Our results demonstrate that the response of NG2(+) cells in the SVZ and their subsequent differentiation in CC after focal demyelination depend on EGFR signaling.
Figures
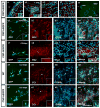
Figure 1. NG2-expressing cells are present in the SVZ and migrate out to the CC after focal demyelination
(a) Confocal images of immunostaining in the CNP-hEGFR mouse brain at P60 with anti-NG2. A large percentage of NG2+ cells are present in the ependymal layer of the SVZ (a1, epl-SVZ), anterior subventricular zone (a2, aSVZ), corpus callosum (a3, CC) and cortex (a4, Ctx), displaying different morphologies. (a5) NIT-GFP retrovirus was injected into the adult SVZ 2 days prior to LPC injection. GFP+ cells were detected migrating out of the SVZ, and a significant number of GFP+ cells were observed in the lesioned area of the CC of the CNP-hEGFR mouse at 5dpl (a5). (b1–b4) At 2dpl, a significant percentage of infected GFP+ cells in the SVZ express NG2 (red, b2) and none of the GFP+ cells was GFAP+ (blue, b3). (c1–c4) At 5dpl, a significant number of virus-infected GFP+ cells were found in CC and a large percentage still expressed NG2 (c2 and c4, red). (d-e) At 10 dpl, the majority of GFP+ cells found in the lesioned CC express markers for mature glia, including CC1 and S100b. However, in CNP-hEGFR mice, the percentage of CC1+ oligodendrocytes generated from virus-infected cells was higher than in WT mice. Arrows indicate triple-labeled cells. Inserts show immunostaining for the individual antigens for cells indicated by arrows. Scale bar = 50μm
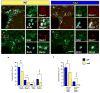
Figure 2. Reduced NG2+Mash1+Olig2+ progenitor cells in the adult SVZ of the wa2 mouse
(a and b) Triple labeling with anti-Olig2 (green), anti-Mash1 (red), and BrdU (blue) in the SVZ of WT (a) and Wa2 (b) mice at P60. (c and d) Triple labeling with anti-NG2 (green), anti-Mash1 (red), and BrdU (blue) in the SVZ of WT (c) and wa2 (d) mice at P60. Scale bar = 50μm. (e) Total Olig2+, Mash1+, NG2+, and BrdU+ cells in the SVZ of WT and wa2 mice at P60. (f) Total Olig2+Mash1+BrdU+ and NG2+Mash1+BrdU+ cells in the SVZ of WT and wa2 mice at P60. Data are shown as means ± S.E.M. (n=2–3 brains for each phenotype). ***p<0.001, ** p<0.002 and *p<0.01 paired t-test wa2 vs. WT.
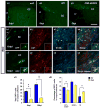
Figure 3. Defect in migration of neural progenitors from the SVZ to the CC of the wa2 mouse after focal demyelination
NIT-GFP retrovirus was injected into the adult SVZ of wa2 (a1), WT (a2), and CNP-hEGFR (a3) mice 2 days prior to LPC injection. At 2dpl, a large percentage of GFP+ infected cells were observed in the WT and CNP-hEGFR mice, but not in the SVZ of the wa2 mouse. (b) At 10dpl, GFP+ infected cells were still found around the SVZ/LV in the wa2 mouse (b1–b4), and a very small percentage reached the lesioned CC (c1–c4 and d1). (d1) At 5 and 10dpl, the total number of virus-infected GFP+ cells found in CC was higher in WT than in wa2 mice. (d2) At 10 dpl, CC1+ oligodendrocytes and GFAP+ astrocytes were observed both in WT and wa2 mice, however the number of differentiated cells was significantly higher in WT than in wa2 mouse. Insert panels show immunostaining for the individual antigens for cells indicated by arrows. NI = not identified - cells that were not CC1+, S100+ or GFAP+. *p<001; **p<0.05. Scale bar = 50μm
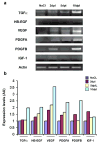
Figure 4. EGFR ligand expression is induced in the SVZ after focal demyelination of the CC
(a) RT-PCR analysis using specific primers for mouse TGFα, HB-EGF, VEGF, PDGFA, PDGFB, IGF1 and Actin. Growth factors expression levels were detected using total RNA from the SVZ after demyelination of the CC at 2dpl, 5dpl and 10dpl. Controls were from NaCl-injected brains at 5dpl. (b) Histograms were obtained by expressing results as percentage of the saline-injected control values.
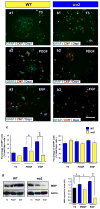
Figure 5. EGF promotes oligodendrogenesis from SVZ neural progenitor cells in culture, and its effect is attenuated in wa2 cells
P15 SVZ tissue was dissociated into single cells and plated onto glass coverslips. Cells were cultured for 4 days in vitro (DIV) in stem cell medium with daily additions of T3, PDGF or EGF, as indicated. After 4DIV, cells were fixed and processed for immunocytochemistry, or were harvested for biochemical analysis. (a and b) Representative images of differentiated cells in WT (a) and wa2 (b) cultures. Cells were stained with anti-CNP (red), anti-GFAP (green) and DAPI (blue). Scale bar = 50μm. (c) Percentage of differentiated glial phenotypes derived from WT and wa2 cells. Data are expressed as percentage of total DAPI+ cells Approximately 300 total cells were counted for each antigen. *p<0.05; **p<0.03. (d) Western blot analysis of MBP expression in SVZ cells from WT and wa2 mice cultured in T3, PDGF or EGF for 4 days. EGF enhances MBP expression in WT, but not in wa2 cells, and is more effective than T3 or PDGF. *p<0.05; **p<0.001.
Similar articles
- Microglial Activation Induces Generation of Oligodendrocyte Progenitor Cells from the Subventricular Zone after Focal Demyelination in the Corpus Callosum.
Naruse M, Shibasaki K, Shimauchi-Ohtaki H, Ishizaki Y. Naruse M, et al. Dev Neurosci. 2018;40(1):54-63. doi: 10.1159/000486332. Epub 2018 Jan 25. Dev Neurosci. 2018. PMID: 29393205 - Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes.
Gonzalez-Perez O, Romero-Rodriguez R, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Gonzalez-Perez O, et al. Stem Cells. 2009 Aug;27(8):2032-43. doi: 10.1002/stem.119. Stem Cells. 2009. PMID: 19544429 Free PMC article. - A functional role for EGFR signaling in myelination and remyelination.
Aguirre A, Dupree JL, Mangin JM, Gallo V. Aguirre A, et al. Nat Neurosci. 2007 Aug;10(8):990-1002. doi: 10.1038/nn1938. Epub 2007 Jul 8. Nat Neurosci. 2007. PMID: 17618276 - The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain.
Galvez-Contreras AY, Quiñones-Hinojosa A, Gonzalez-Perez O. Galvez-Contreras AY, et al. Front Cell Neurosci. 2013 Dec 17;7:258. doi: 10.3389/fncel.2013.00258. Front Cell Neurosci. 2013. PMID: 24381541 Free PMC article. Review. - Adult Neoneurogenesis and Oligodendrogenesis in Multiple Sclerosis: A Systematic Review of Human and Animal Studies.
Liampas A, Tseriotis VS, Artemiadis A, Zis P, Argyropoulou C, Grigoriadis N, Hadjigeorgiou GM, Vavougyios G. Liampas A, et al. Brain Connect. 2024 May;14(4):209-225. doi: 10.1089/brain.2023.0081. Epub 2024 Apr 16. Brain Connect. 2024. PMID: 38534961
Cited by
- Thymosin beta 4 up-regulates miR-200a expression and induces differentiation and survival of rat brain progenitor cells.
Santra M, Chopp M, Santra S, Nallani A, Vyas S, Zhang ZG, Morris DC. Santra M, et al. J Neurochem. 2016 Jan;136(1):118-32. doi: 10.1111/jnc.13394. Epub 2015 Nov 10. J Neurochem. 2016. PMID: 26466330 Free PMC article. - NG2-glia and their functions in the central nervous system.
Dimou L, Gallo V. Dimou L, et al. Glia. 2015 Aug;63(8):1429-51. doi: 10.1002/glia.22859. Epub 2015 May 24. Glia. 2015. PMID: 26010717 Free PMC article. Review. - N-cadherin promotes recruitment and migration of neural progenitor cells from the SVZ neural stem cell niche into demyelinated lesions.
Klingener M, Chavali M, Singh J, McMillan N, Coomes A, Dempsey PJ, Chen EI, Aguirre A. Klingener M, et al. J Neurosci. 2014 Jul 16;34(29):9590-606. doi: 10.1523/JNEUROSCI.3699-13.2014. J Neurosci. 2014. PMID: 25031401 Free PMC article. Retracted. - Tissue-type plasminogen activator exerts EGF-like chemokinetic effects on oligodendrocytes in white matter (re)myelination.
Leonetti C, Macrez R, Pruvost M, Hommet Y, Bronsard J, Fournier A, Perrigault M, Machin I, Vivien D, Clemente D, De Castro F, Maubert E, Docagne F. Leonetti C, et al. Mol Neurodegener. 2017 Feb 23;12(1):20. doi: 10.1186/s13024-017-0160-5. Mol Neurodegener. 2017. PMID: 28231842 Free PMC article. - Role of the cellular prion protein in oligodendrocyte precursor cell proliferation and differentiation in the developing and adult mouse CNS.
Bribián A, Fontana X, Llorens F, Gavín R, Reina M, García-Verdugo JM, Torres JM, de Castro F, del Río JA. Bribián A, et al. PLoS One. 2012;7(4):e33872. doi: 10.1371/journal.pone.0033872. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22529900 Free PMC article.
References
- Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nature Neuroscience. 2007;10:990–1002. - PubMed
- Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. Journal of Neuroscience Research. 1990;27:400–407. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous