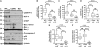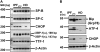Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis - PubMed (original) (raw)
Comparative Study
. 2008 Oct 15;178(8):838-46.
doi: 10.1164/rccm.200802-313OC. Epub 2008 Jul 17.
Clemens Ruppert, Poornima Mahavadi, Ingrid Henneke, Philipp Markart, Miriam Koch, Gyoergy Lang, Ludger Fink, Rainer-Maria Bohle, Werner Seeger, Timothy E Weaver, Andreas Guenther
Affiliations
- PMID: 18635891
- PMCID: PMC2566794
- DOI: 10.1164/rccm.200802-313OC
Comparative Study
Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis
Martina Korfei et al. Am J Respir Crit Care Med. 2008.
Abstract
Rationale: The molecular pathomechanisms underlying idiopathic pulmonary fibrosis (IPF) are elusive, but chronic epithelial injury has recently been suggested as key event.
Objectives: We investigated the possible implication of endoplasmic reticulum (ER) stress-mediated apoptosis in sporadic IPF.
Methods: We analyzed peripheral explanted lung tissues from patients with sporadic IPF (n = 24), chronic obstructive pulmonary disease (COPD) (n = 9), and organ donors (n = 12) for expression of major ER stress mediators and apoptosis markers by means of immunoblotting, semiquantitative reverse transcription-polymerase chain reaction, immunohistochemistry, and the TUNEL method.
Measurements and main results: Compared with COPD and donor lungs, protein levels of ER stress mediators, such as processed p50 activating transcription factor (ATF)-6 and ATF-4 and the apoptosis-inductor CHOP (C/EBP-homologous protein), as well as transcript levels of spliced X-box binding protein (XBP)-1, were significantly elevated in lung homogenates and type II alveolar epithelial cells (AECIIs) of IPF lungs. Proapoptotic, oligomeric forms of Bax, which play a key role in ER stress-mediated apoptosis downstream of CHOP induction, as well as caspase-3 cleavage, could be detected in IPF lungs. By means of immunohistochemistry, exclusive induction of active ATF-6, ATF-4, and CHOP in AECIIs was encountered in IPF but not in COPD or donor lungs. Immunoreactivity was most prominent in the epithelium near dense zones of fibrosis and fibroblast foci, where these ER stress markers colocalized with markers of apoptosis (TUNEL, cleaved caspase-3).
Conclusions: Severe ER stress response in the AECIIs of patients with sporadic IPF may underlie the apoptosis of this cell type and development of fibrosis in this disease.
Figures
**Figure 1.
Induction of the endoplasmic reticulum stress–mediated apoptosis pathway in idiopathic pulmonary fibrosis (IPF). (A) Example immunoblots and (B–F) quantitative immunoblot analysis of peripheral lung tissue from patients with sporadic IPF (IPFLTX) (n = 20), lung tissue from patients with chronic obstructive pulmonary disease (COPD) (n = 9), and human donor (HD) lungs (n = 12) using specific antibodies against activating transcription factor (ATF)-6α (B), ATF-4 (C), CHOP (D), Bax (E), pro–caspase 3 (F), and β-actin as loading control. Densitometric ratios of the respective protein to β-actin are depicted as a box-and-whisker diagram (box indicates 25th and 75th, horizontal line indicates the 50th percentile [median], and extensions above and below reflect extreme values). Measurements of individual samples were done in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001. ns = not significant.
**Figure 2.
X-box binding protein (XBP)-1 activation in idiopathic pulmonary fibrosis (IPF) lungs. Total RNA was isolated from peripheral lung tissue of patients with IPF (IPFLTX), from lung tissue of patients with chronic obstructive pulmonary disease (COPD), and human donors (HD) and subjected to reverse transcription–polymerase chain reaction analysis with primers spanning the intron of unspliced XBP-1 mRNA. XBP-1(u) denotes unspliced, inactive XBP-1; XBP-1(s) denotes spliced, active XBP-1, which was observed in all 20 IPFLTX lungs, but in none of the COPD or HD lungs. Representative results are shown. MW = molecular weight.
**Figure 3.
Apoptotic endoplasmic reticulum stress response in alveolar epithelial type II cells (AECIIs) in idiopathic pulmonary fibrosis (IPF). (A) Reverse transcription–polymerase chain reaction of human AECIIs isolated from peripheral explanted lung tissue (LTX) of patients with IPF (n = 3) and healthy donors (HD) (n = 3) demonstrated strong induction of C/EBP-homologous protein (CHOP) expression and exclusive splicing of the X-box binding protein (XBP)-1 transcript in IPF; in contrast, expression of surfactant protein (SP)-B, SP-C, thyroid transcription factor 1 (TTF-1) and β-actin was similar in IPF and donor lungs. (B) Immunoblot analysis of human AECIIs isolated from peripheral LTX lung tissue of patients with IPF (n = 2) and HD (n = 3) revealed up-regulated protein expression of Bip, ATF-4, and CHOP in IPF. Loading control: β-actin. MW = molecular weight.
**Figure 4.
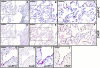
Up-regulation of activating transcription factor (ATF)-6 in alveolar epithelial type II cells (AECIIs) of idiopathic pulmonary fibrosis (IPF) lungs. Representative immunohistochemistry for pro–surfactant protein (SP)-C in IPF (A, C, E, G) and donor (I, K) lung tissues (red stain indicates AECIIs) and for ATF-6 in serial sections of IPF (B, D, F, H) and donor (J, L) lung tissues. (B, D, F, H) In IPF, localization of ATF-6 was exclusively observed in AECIIs surrounded by fibrotic tissue (B and D, violet stain), in AECIIs of thickened alveolar septae (B and F, arrows), and in AECIIs of histologically normal appearing areas of IPF lung parenchyma (B and H, arrows). AECIIs near dense zones of fibrosis revealed a very strong staining for ATF-6 (B and D). (J, L) No notable staining for ATF6 was observed in any cell of the donor tissues. Original magnification of photomicrographs A and B: ×100 (bar = 200 μm); original magnification of photomicrographs C_–_H, K, L: ×400 (bar = 50 μm); original magnification of photomicrographs I and J: ×200 (bar = 100 μm).
**Figure 5.
Colocalization of pro–surfactant protein (SP)-C, activating transcription factor (ATF)-6, and activated caspase 3 in alveolar epithelial type-II cells (AECIIs) in areas of dense fibrosis in idiopathic pulmonary fibrosis (IPF). Representative immunohistochemistry for pro–SP-C (A_–_C), ATF-6 (D_–_F), and cleaved caspase 3 (G_–_I) in serial sections of IPF lung tissues. AECIIs covering dense areas of fibrosis (A, B) and fibroblast foci (C) showed robust staining for ATF-6 (D–F, arrows) and caspase-3 activation (G–I, arrows). Some pro–SP-C, ATF-6, and caspase-3–positive cells were also detected in the airspaces, presumably representing detached apoptotic AECIIs, prone to the fate of phagocytosis by alveolar macrophages (asterisks in C, F, and J). Original magnification of photomicrographs A, B, D, E, G, and H: ×400 (bar = 50 μm); original magnification of photomicrographs C, F, and I: ×200 (bar = 100 μm).
**Figure 6.
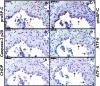
Induction of C/EBP-homologous protein (CHOP) in alveolar epithelial type II cells (AECIIs) of idiopathic pulmonary fibrosis (IPF) lungs. Representative immunohistochemistry for pro–surfactant protein (SP)-C in IPF (A_–_D, I, J) and donor (M, N) lung tissues (red stain, arrows) and for CHOP in serial sections of IPF (E_–_H, K, L) and donor (O, P) lung tissues (pale pink stain, arrows). In IPF, CHOP stained the nucleus and cytoplasm of hyperplastic AECIIs covering zones of overt fibrosis (E_–_H) and fibroblastic foci (K, L). Positive staining for CHOP was not seen in any cell and in any of the donor lung tissues (O, P). Original magnification of photomicrographs A, C, E, G, I, K, M, and O: ×200 (bar = 100 μm); original magnification of photomicrographs B, D, F, H, J, L, N, and P: ×400 (bar = 50 μm).
**Figure 7.
Induction of C/EBP-homologous protein (CHOP) in alveolar epithelial type II cells (AECIIs) of histologically normal appearing areas of idiopathic pulmonary fibrosis (IPF) lung parenchyma. Representative immunohistochemistry for pro–surfactant protein (SP)-C (A_–_C, G, I) and for CHOP (D_–_F, H, J) in serial sections of IPF lung tissue. In AECIIs of histologically normal appearing areas of IPF lung parenchyma, CHOP induction was observed to a lesser extent (D_–_F; arrows indicate CHOP-positive AECIIs) than in AECIIs lining airspaces near dense fibrotic zones (H, J). Photomicrographs A_–_C, G, I, D_–_F, H, and J are from the same IPF lung tissue section. Original magnification of photomicrographs A and D: ×100 (bar = 200 μm); original magnification of photomicrographs B, E, G, and H: ×200 (bar = 100 μm); original magnification of photomicrographs C, F, I, and J: ×400 (bar = 50 μm).
**Figure 8.
“Hyperplastic” alveolar epithelial type II cells (AECIIs) show severe endoplasmic reticulum stress and consecutive apoptosis. Colocalization of pro–surfactant protein (SP)-C, activated caspase 3, C/EBP-homologous protein (CHOP), activating transcription factor (ATF)-6, and ATF-4 in AECIIs covering fibroblastic foci. Representative immunohistochemistry for pro–SP-C (A, D), cleaved caspase 3 (B), CHOP (C), ATF-6 (E), and ATF-4 (F) in serial sections of idiopathic pulmonary fibrosis lung tissue. Original magnification of photomicrographs A_–_F: ×400 (bar = 50 μm).
Comment in
- Stress in the ER (endoplasmic reticulum): a matter of life and death for epithelial cells.
Horowitz JC, Limper AH. Horowitz JC, et al. Am J Respir Crit Care Med. 2008 Oct 15;178(8):782-3. doi: 10.1164/rccm.200807-1138ED. Am J Respir Crit Care Med. 2008. PMID: 18832553 Free PMC article. No abstract available.
Similar articles
- Endoplasmic reticulum stress-mediated apoptotic pathway is involved in corpus luteum regression in rats.
Yang Y, Sun M, Shan Y, Zheng X, Ma H, Ma W, Wang Z, Pei X, Wang Y. Yang Y, et al. Reprod Sci. 2015 May;22(5):572-84. doi: 10.1177/1933719114553445. Epub 2014 Oct 20. Reprod Sci. 2015. PMID: 25332219 Free PMC article. - Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection.
Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, Miller GG, Loyd JE, Blackwell TS. Lawson WE, et al. Am J Physiol Lung Cell Mol Physiol. 2008 Jun;294(6):L1119-26. doi: 10.1152/ajplung.00382.2007. Epub 2008 Apr 4. Am J Physiol Lung Cell Mol Physiol. 2008. PMID: 18390830 - Endoplasmic reticulum stress as a correlate of cytotoxicity in human tumor cells exposed to diindolylmethane in vitro.
Sun S, Han J, Ralph WM Jr, Chandrasekaran A, Liu K, Auborn KJ, Carter TH. Sun S, et al. Cell Stress Chaperones. 2004 Mar;9(1):76-87. doi: 10.1379/csc-2r.1. Cell Stress Chaperones. 2004. PMID: 15270080 Free PMC article. - Lost after translation: insights from pulmonary surfactant for understanding the role of alveolar epithelial dysfunction and cellular quality control in fibrotic lung disease.
Mulugeta S, Nureki S, Beers MF. Mulugeta S, et al. Am J Physiol Lung Cell Mol Physiol. 2015 Sep 15;309(6):L507-25. doi: 10.1152/ajplung.00139.2015. Epub 2015 Jul 17. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26186947 Free PMC article. Review. - Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis.
Tanjore H, Blackwell TS, Lawson WE. Tanjore H, et al. Am J Physiol Lung Cell Mol Physiol. 2012 Apr 15;302(8):L721-9. doi: 10.1152/ajplung.00410.2011. Epub 2012 Jan 27. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22287606 Free PMC article. Review.
Cited by
- Increased Levels of ER Stress and Apoptosis in a Sheep Model for Pulmonary Fibrosis Are Alleviated by In Vivo Blockade of the KCa3.1 Ion Channel.
Perera UE, Organ L, Dewage SNV, Derseh HB, Stent A, Snibson KJ. Perera UE, et al. Can Respir J. 2021 Mar 19;2021:6683195. doi: 10.1155/2021/6683195. eCollection 2021. Can Respir J. 2021. PMID: 33828632 Free PMC article. - Inhibition of the cGAS-STING Pathway Attenuates Lung Ischemia/Reperfusion Injury via Regulating Endoplasmic Reticulum Stress in Alveolar Epithelial Type II Cells of Rats.
Huang R, Shi Q, Zhang S, Lin H, Han C, Qian X, Huang Y, Ren X, Sun J, Feng N, Xia C, Shi M. Huang R, et al. J Inflamm Res. 2022 Sep 5;15:5103-5119. doi: 10.2147/JIR.S365970. eCollection 2022. J Inflamm Res. 2022. PMID: 36091334 Free PMC article. - The UPR and lung disease.
Osorio F, Lambrecht B, Janssens S. Osorio F, et al. Semin Immunopathol. 2013 May;35(3):293-306. doi: 10.1007/s00281-013-0368-6. Epub 2013 Mar 28. Semin Immunopathol. 2013. PMID: 23536202 Review. - New Insights via RNA Profiling of Formalin-Fixed Paraffin-Embedded Lung Tissue of Pulmonary Fibrosis Patients.
Klay D, Kazemier KM, van der Vis JJ, Smits HM, Grutters JC, van Moorsel CHM. Klay D, et al. Int J Mol Sci. 2023 Nov 25;24(23):16748. doi: 10.3390/ijms242316748. Int J Mol Sci. 2023. PMID: 38069069 Free PMC article. - Oxidative stress and pulmonary fibrosis.
Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Cheresh P, et al. Biochim Biophys Acta. 2013 Jul;1832(7):1028-40. doi: 10.1016/j.bbadis.2012.11.021. Epub 2012 Dec 5. Biochim Biophys Acta. 2013. PMID: 23219955 Free PMC article. Review.
References
- Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 1994;150:967–972. - PubMed
- American Thoracic Society; European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. - PubMed
- Noble PW, Homer RJ. Back to the future: historical perspective on the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol 2005;33:113–120. - PubMed
- Corrin B, Dewar A, Rodriguez-Roisin R, Turner-Warwick M. Fine structural changes in cryptogenic fibrosing alveolitis and asbestosis. J Pathol 1985;147:107–119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials