Cutting edge: The transmembrane E3 ligase GRAIL ubiquitinates the costimulatory molecule CD40 ligand during the induction of T cell anergy - PubMed (original) (raw)
Cutting edge: The transmembrane E3 ligase GRAIL ubiquitinates the costimulatory molecule CD40 ligand during the induction of T cell anergy
Neil B Lineberry et al. J Immunol. 2008.
Abstract
Activation of naive T lymphocytes is regulated through a series of discrete checkpoints that maintain unresponsiveness to self. During this multistep process, costimulatory interactions act as inducible signals that allow APCs to selectively mobilize T cells against foreign Ags. In this study, we provide evidence that the anergy-associated E3 ubiquitin ligase GRAIL (gene related to anergy in lymphocytes) regulates expression of the costimulatory molecule CD40L on CD4 T cells. Using its luminal protease-associated domain, GRAIL binds to the luminal/extracellular portion of CD40L and facilitates transfer of ubiquitin molecules from the intracellular GRAIL RING (really interesting new gene) finger to the small cytosolic portion of CD40L. Down-regulation of CD40L occurred following ectopic expression of GRAIL in naive T cells from CD40(-/-) mice, and expression of GRAIL in bone marrow chimeric mice was associated with diminished lymphoid follicle formation. These data provide a model for intrinsic T cell regulation of costimulatory molecules and a molecular framework for the initiation of clonal T cell anergy.
Conflict of interest statement
Disclosures: The authors have no financial conflict of interest.
Figures
FIGURE 1
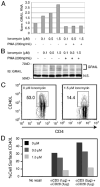
Ionomycin-up-regulated GRAIL expression is associated with reduced CD40L expression. A and B, MACS-purified CD4 T cells from BALB/c mice were anergized with a low (0.1 _μ_M), medium (0.5 _μ_M), or high (1.5 _μ_M) ionomycin dose with or without PMA (200 ng/ml) overnight. Cells were then harvested for QPCR (A) or Western blot analysis (B) of GRAIL expression. N.S., nonspecific band used as a loading control; IB, immunoblot; Norm., normalized. C, MACS-purified BALB/c CD4 T cells were anergized with 0 _μ_M (left) or 1.5 _μ_M (right) ionomycin overnight. Live cells were then washed, stimulated, and stained for cell surface CD40L. D, Histograms representing the percentage of cell surface CD40L expressed on gated CD4 T cells with varying conditions of ionomycin anergy treatment and recall stimulation. Black histogram represents CD4 T cells treated with 0 _μ_M ionomycin, dark gray histogram denotes CD4 T cells treated with 0.5 _μ_M ionomycin, and light gray histogram represents CD4 T cells treated with 1.5 _μ_M ionomycin.
FIGURE 2
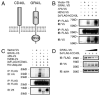
GRAIL binds and ubiquitinates CD40L. A, Schematic display of the domains and orientation of GRAIL and CD40L. B, 293T cells were transfected with 3× FLAG-tagged human CD40L (hCD40L) and the indicated V5-tagged GRAIL constructs. Eluted proteins from anti-FLAG conjugated agarose were separated by SDS-PAGE and blotted with the indicated Abs. IB, Immunoblot; IP, immunoprecipitation. C, 293T cells were transfected with 3× FLAG-tagged hCD40L, V5-tagged GRAIL constructs, and HA-tagged ubiquitin. Eluted proteins from anti-FLAG-conjugated agarose were separated by SDS-PAGE and blotted with the indicated Abs. D, 293T cells were transfected with 0.2 μg of 3× FLAG-tagged hCD40L and V5-tagged GRAIL at 0.4, 0.8, 1.2, and 1.6 μg (empty V5 vector was added for a total of 2 μg of total V5 plasmid for each transfection). Cell lysates were separated by SDS-PAGE and blotted with the indicated Abs.
FIGURE 3
GRAIL expression down-regulates endogenous CD40L expression. A, Freshly MACS-purified CD4 T cells from C57BL/6 and CD40−/− mice were stained for CD40L. Gray histogram represents unstimulated wild-type CD4 T cells, thick line denotes live CD40−/− CD4 T cells, and dotted line represents intracellular staining of CD40−/− CD4 T cells. B, MACS purified CD4 T cells from CD40−/− mice were stimulated overnight in vitro and then infected with retrovirus expressing the indicated GRAIL construct. After 36 h, cells were stained for total CD40L and gated GFP+ cells are shown. Thick line represents vector-transduced CD4 T cells, gray line denotes GRAIL transduced CD4 T cells, and dotted line represents ΔPA-transduced CD4 T cells.
FIGURE 4
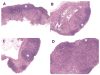
GRAIL expression in naive CD4 T cells results in diminished follicle formation in vivo. DO11.10 bone marrow chimeric mice were generated as described (6). Mice were sacrificed 28 days after injection of transduced hematopoietic cells and lymphoid tissue was processed for histological analysis. A, GFP vector control. B, Wild-type GRAIL. C, Dominant negative H2N2 GRAIL. D, Otubain-1 ARF-1 (epistatic protein stabilizer of GRAIL). Original magnification was × 10;✩ denotes follicular zone. One representative mouse is shown from two to three mice per group from three independent experiments.
Similar articles
- The transmembrane E3 ligase GRAIL ubiquitinates and degrades CD83 on CD4 T cells.
Su LL, Iwai H, Lin JT, Fathman CG. Su LL, et al. J Immunol. 2009 Jul 1;183(1):438-44. doi: 10.4049/jimmunol.0900204. J Immunol. 2009. PMID: 19542455 Free PMC article. - The single subunit transmembrane E3 ligase gene related to anergy in lymphocytes (GRAIL) captures and then ubiquitinates transmembrane proteins across the cell membrane.
Lineberry N, Su L, Soares L, Fathman CG. Lineberry N, et al. J Biol Chem. 2008 Oct 17;283(42):28497-505. doi: 10.1074/jbc.M805092200. Epub 2008 Aug 18. J Biol Chem. 2008. PMID: 18713730 Free PMC article. - E3 ubiquitin ligase GRAIL controls primary T cell activation and oral tolerance.
Kriegel MA, Rathinam C, Flavell RA. Kriegel MA, et al. Proc Natl Acad Sci U S A. 2009 Sep 29;106(39):16770-5. doi: 10.1073/pnas.0908957106. Epub 2009 Sep 17. Proc Natl Acad Sci U S A. 2009. PMID: 19805371 Free PMC article. - E3 ubiquitin ligases as T cell anergy factors.
Mueller DL. Mueller DL. Nat Immunol. 2004 Sep;5(9):883-90. doi: 10.1038/ni1106. Nat Immunol. 2004. PMID: 15334084 Review. - The role of CD40 ligand in costimulation and T-cell activation.
Grewal IS, Flavell RA. Grewal IS, et al. Immunol Rev. 1996 Oct;153:85-106. doi: 10.1111/j.1600-065x.1996.tb00921.x. Immunol Rev. 1996. PMID: 9010720 Review.
Cited by
- E3 Ubiquitin Ligases as Immunotherapeutic Target in Atherosclerotic Cardiovascular Disease.
Poels K, Vos WG, Lutgens E, Seijkens TTP. Poels K, et al. Front Cardiovasc Med. 2020 Jun 5;7:106. doi: 10.3389/fcvm.2020.00106. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32582770 Free PMC article. Review. - Does antigen masking by ubiquitin chains protect from the development of autoimmune diseases?
Weil R. Weil R. Front Immunol. 2014 Jun 3;5:262. doi: 10.3389/fimmu.2014.00262. eCollection 2014. Front Immunol. 2014. PMID: 24917867 Free PMC article. Review. - Gut commensal bacteria enhance pathogenesis of a tumorigenic murine retrovirus.
Spring J, Khan AA, Lara S, O'Grady K, Wilks J, Gurbuxani S, Erickson S, Fischbach M, Jacobson A, Chervonsky A, Golovkina T. Spring J, et al. Cell Rep. 2022 Sep 13;40(11):111341. doi: 10.1016/j.celrep.2022.111341. Cell Rep. 2022. PMID: 36103821 Free PMC article. - Ethylenecarbodiimide-treated splenocytes carrying male CD4 epitopes confer histocompatibility Y chromosome antigen transplant protection by inhibiting CD154 upregulation.
Martin AJ, McCarthy D, Waltenbaugh C, Goings G, Luo X, Miller SD. Martin AJ, et al. J Immunol. 2010 Sep 15;185(6):3326-36. doi: 10.4049/jimmunol.1000802. Epub 2010 Aug 16. J Immunol. 2010. PMID: 20713889 Free PMC article. - Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD4+ 8- thymocytes and invariant NKT cells but not in Treg cells.
Koguchi Y, Buenafe AC, Thauland TJ, Gardell JL, Bivins-Smith ER, Jacoby DB, Slifka MK, Parker DC. Koguchi Y, et al. PLoS One. 2012;7(2):e31296. doi: 10.1371/journal.pone.0031296. Epub 2012 Feb 21. PLoS One. 2012. PMID: 22363608 Free PMC article.
References
- Schwartz RH. Tcell anergy. Annu Rev Immunol. 2003;21:305–334. - PubMed
- Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. - PubMed
- Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. - PubMed
- Mueller DL. E3 ubiquitin ligases as T cell anergy factors. Nat Immunol. 2004;5:883–890. - PubMed
- Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- CA 65237-17/CA/NCI NIH HHS/United States
- T32-AI07290-21/AI/NIAID NIH HHS/United States
- R01 CA065237/CA/NCI NIH HHS/United States
- U19-AI070352/AI/NIAID NIH HHS/United States
- U19 AI070352/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous