p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation - PubMed (original) (raw)
p120-Catenin regulates leukocyte transmigration through an effect on VE-cadherin phosphorylation
Pilar Alcaide et al. Blood. 2008.
Abstract
Vascular endothelial-cadherin (VE-cad) is localized to adherens junctions at endothelial cell borders and forms a complex with alpha-, beta-, gamma-, and p120-catenins (p120). We previously showed that the VE-cad complex disassociates to form short-lived "gaps" during leukocyte transendothelial migration (TEM); however, whether these gaps are required for leukocyte TEM is not clear. Recently p120 has been shown to control VE-cad surface expression through endocytosis. We hypothesized that p120 regulates VE-cad surface expression, which would in turn have functional consequences for leukocyte transmigration. Here we show that endothelial cells transduced with an adenovirus expressing p120GFP fusion protein significantly increase VE-cad expression. Moreover, endothelial junctions with high p120GFP expression largely prevent VE-cad gap formation and neutrophil leukocyte TEM; if TEM occurs, the length of time required is prolonged. We find no evidence that VE-cad endocytosis plays a role in VE-cad gap formation and instead show that this process is regulated by changes in VE-cad phosphorylation. In fact, a nonphosphorylatable VE-cad mutant prevented TEM. In summary, our studies provide compelling evidence that VE-cad gap formation is required for leukocyte transmigration and identify p120 as a critical intracellular mediator of this process through its regulation of VE-cad expression at junctions.
Figures
Figure 1
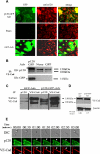
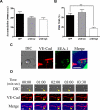
Characterization of p120 expression in vascular endothelium and p120GFP/VE-cad gap formation during leukocyte TEM at the endothelial cell junctions. Confluent HUVECs were transduced with GFP or p120GFP adenovirues as described in “Adenovirus production and cell infection.” (A) p120GFP colocalizes with endogenous p120 at cell junctions. Monolayers were transduced with p120GFP, or sham treated, fixed with 10% buffered formalin, permeabilized, and stained with anti-p120 mAb. Representative fields were examined by epifluorescence microscopy and show junctional distribution of p120GFP and colocalization with endogenous p120 at cell junctions. (B) VE-cad was immunoprecipitated from the HUVEC lysates, and the material was immunoblotted for p120 to show endogenous isoforms of p120/p100, or with an anti-GFP mAb to detect expression of p120GFP. (C) HUVECs were surface biotinylated, lysed, and subjected to immunoprecipitation with anti-p120 mAb, or VE-cad mAb. The association of p120GFP with VE-cad was detected with streptavidin-peroxidase. Vertical lines have been inserted to indicate a repositioned gel lane. (D) Transduced HUVECs were lysed directly and blotted with Hec-1 to detect total VE-cad. Normalized VE-cad values versus β-actin are shown by OD numbers. Data are representative of 3 separate studies. (E) Three-channel live-time microscopy of PMNs in the process of transmigration. Paired 2-color fluorescence of VE-cad Alexa 568–stained HUVECs (red channel), p120GFP low-dose infected HUVECs (green channel), and simultaneous DIC images are presented. At t = 0, PMN approaches the brightly stained cell junction. At t = 1:00, PMN starts to transmigrate and a de novo gap is detected. At t = 3:00, the gap is sealed. This gap in p120-catenin GFP colocalized with the gap formed by VE-cadherin (red) during transmigration, as demonstrated in the merge panel. Bar represents 10 μm. The figure represents a typical sequence of events during PMN TEM. Five independent experiments using HUVECs and PMNs from multiple donors were analyzed.
Figure 2
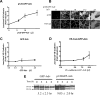
p120GFP overexpression increases VE-cad at the cell-cell junctions. HUVECs were infected with different doses of p120GFP (A,B), GFP (C), or VE-cadherin GFP (D). Hec-1–Alexa 568 Ab was used to detect VE-cad in each monolayer. The intensity of VE-cad at the junctions was quantified in live HUVECs by analyzing 20 cell-cell junctions including representative junctions of the heterogeneous population for each condition (in duplicate) for each experiment performed. (E) HUVECs were transduced with GFP or p120GFP (17.5 μL) surface biotinylated and lysed, or cultured for 2, 4, or 6 hours before being lysed and subjected to immunoprecipitation with Hec-1. A representative blot is shown. Values corresponding to the half-life were calculated according to the “exponential decay formula” and are the mean plus or minus SD of 3 different experiments.
Figure 3
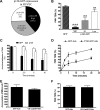
Overexpression of p120 in HUVECs inhibits PMN transmigration. (A) The level of p120 GFP fluorescence and PMN transmigration was quantified as described in “Image acquisition and analysis” and “Quantitation of p120GFP and VE-cad fluorescence in endothelium,” using live cell fluorescence digital imaging. The p120GFP expression was grouped into 3 categories (low, intermediate, and high). (B) The amount of TEM was determined in each category. Values represent the mean plus or minus SD of 5 different experiments. P values are indicated, comparing each bar with GFP construct. ***P < .001; **P < .01. (C) PMNs remained bound at the cell-cell junctions on monolayers overexpressing p120GFP, but disappeared from the cell-cell junctions and efficiently transmigrated when perfused on HUVECs transduced with GFP. Values represent (number of cells bound to the junctions)/(number of total cells bound) × 100, and are the mean plus or minus SD of 5 different experiments. *P < .01. (D) The inhibitory effect of p120 overexpression was not due to a delay in PMN transmigration, as the block of TEM was sustained for more than 20 minutes. The percentage of TEM was normalized by dividing percentage of TEM at each time point by the number of cells that were bound initially at time 0. P values for p120GFP versus GFP at each time point are indicated. *P < .05; **P < .01. Data represent mean plus or minus SD of 3 different experiments. (E) Total accumulation of PMNs was similar on VE-cad–GFP– or GFP-transduced monolayers. (F) Overexpression of VE-cadherin GFP did not affect PMN TEM. Values in panels E and F represent the mean plus or minus SD from 3 different experiments using HUVECs and PMNs from different donors.
Figure 4
Overexpression of p120 results in hypophosphorylation of VE-cadherin, which in turn, regulates TEM. (A) HUVEC monolayers were infected with a low or a high dose of p120GFP (6 μL, 17.5 μL in Figure 2A) or GFP (2 μL in Figure 2C), lysed, and subjected to Western blot for phospho–VE-cad–Tyr658, total VE-cad, and β-actin. A representative blot is shown from 4 experiments performed. The graph represents normalized values obtained by densitometry analysis, indicating the relative absorbance of phospho–VE-cadherin–658 with respect to total VE-cadherin for each condition and normalized with the GFP values, and are the mean plus or minus SD of 4 different experiments. β-actin is shown as a loading control. (B) HUVECs were transduced with GFP or a high dose of p120GFP, treated with TNF-α, and incubated with control IgG beads or anti–ICAM-1 beads for 10 minutes and lysed in hot sample buffer. Samples were then diluted to allow immunoprecipitation with 4G10 mAb, and blotted for phospho–VE-cad–Tyr658 or phospho–VE-cad–Tyr731. A representative blot is shown from 3 independent experiments. The graph represents normalized values obtained by densitometry analysis corresponding to phospho–VE-cad divided by the total VE-cad input, and with respect to the GFP-IgG beads value, and is the mean plus or minus SD of 3 separate experiments. *P < .05; **P < .01. (C,D) HUVECs were infected with p120GFP, GFP, VE-cad, or the mutated version of VE-cad at Tyr658 or Tyr731. Overexpression of VE-cad Y658F and Y731F strongly inhibited TEM (C; **P < .01; *P < .05 with respect to WT), whereas total accumulation of PMNs was similar for every condition tested (D). Values represent the mean plus or minus SD from 3 different experiments.
Figure 5
Comparison of constitutive internalization of VE-cad in HUVECs and MVECs. (A) HUVEC or MVEC monolayers were stained with Alexa Fluor 568–conjugated mAb directed against the extracellular domain of VE-cad at 15°C (time 0). Cells were then transferred to 37°C for 3 hours. The location of VE-cad was examined by immunofluorescence microscopy. (B) Upon 3-hour incubation as in panel A, cells were fixed with 4% formaldehyde and stained for EEA-1. Colocalization of VE-cad and EEA-1 was determined by immunofluorescence microscopy, and quantified by counting merged (yellow) vesicles per field. Data represents the total number of merged vesicles per field using a 60× objective, and are the mean plus or minus SD of 2 different samples and 3 different experiments. ***P < .001.
Figure 6
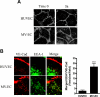
Overexpression of p120 in MVECs inhibits PMN transmigration, and real-time imaging of VE-cad during PMN transmigration in MVECs. (A,B) MVEC monolayers were infected with different doses of AdV-p120GFP or AdV-GFP, and stimulated for 4 hours with TNF-α before the PMNs were perfused. Hec-1—Alexa 568 mAb was used to detect surface VE-cad by immunofluorescence staining. Total accumulation of PMNs was similar on p120GFP- or GFP-transduced monolayers (A). Overexpression of p120GFP (high dose) strongly inhibited TEM (B; P < .001). Values represent the mean plus or minus SD from 3 different experiments. (C,D) TNF-α–activated MVEC monolayers were immunolabeled with VE-cad—Alexa 568 mAb and inserted into the flow chamber. PMNs were perfused for 5 minutes and coverslips were fixed, permeabilized, and stained with EEA-1 Ab (C) or PMNs were allowed to transmigrate for 10 minutes and analyzed by 2-channel live-time microscopy (D). As the neutrophil begins to transmigrate, VE-cad forms a gap at the junction (panel 00:00) that widens (panel 02:00) and reseals (panel 03:00; yellow arrows), without apparent internalization of vesicles of VE-cad or recycling to the membrane of constitutively internalized VE-cad vesicles (thin white arrows). Merge panels show colocalization of the gap and the PMNs during the TEM process. Bar represents 10 μm. The figure represents a typical sequence of events during PMN transmigration from 3 independent experiments using HUVECs and PMNs from multiple donors.
Figure 7
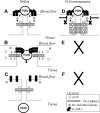
A model that envisions the p120/VE-cad complex as a key regulator of leukocyte TEM. (A) PMN interaction with adhesion molecules such as ICAM-1 on the endothelium surface triggers activation of src and other kinases. (B) Activation of kinases results in phosphorylation of VE-cad, dissociation of p120 from VE-cad, and opening of a junctional gap through which the PMN transmigrates. (C) Upon completion of TEM, p120 binds again to VE-cad as the gap reseals. (D) When p120 is overexpressed, receptor and nonreceptor tyrosine kinases fail to compete with the high levels of p120 present in the cytosol, VE-cad cannot be phosphorylated, and p120 is not released from the VE-cad complex. (E,F) This results in a lack of displacement of VE-cad and lack of gap formation, leading to diminished of PMN TEM.
Similar articles
- p120-Catenin prevents neutrophil transmigration independently of RhoA inhibition by impairing Src dependent VE-cadherin phosphorylation.
Alcaide P, Martinelli R, Newton G, Williams MR, Adam A, Vincent PA, Luscinskas FW. Alcaide P, et al. Am J Physiol Cell Physiol. 2012 Aug 15;303(4):C385-95. doi: 10.1152/ajpcell.00126.2012. Epub 2012 May 30. Am J Physiol Cell Physiol. 2012. PMID: 22648953 Free PMC article. - ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.
Allingham MJ, van Buul JD, Burridge K. Allingham MJ, et al. J Immunol. 2007 Sep 15;179(6):4053-64. doi: 10.4049/jimmunol.179.6.4053. J Immunol. 2007. PMID: 17785844 - Regulation of endothelial barrier function by p120-catenin∙VE-cadherin interaction.
Garrett JP, Lowery AM, Adam AP, Kowalczyk AP, Vincent PA. Garrett JP, et al. Mol Biol Cell. 2017 Jan 1;28(1):85-97. doi: 10.1091/mbc.E16-08-0616. Epub 2016 Nov 16. Mol Biol Cell. 2017. PMID: 27852896 Free PMC article. - Role of p120-catenin in cadherin trafficking.
Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Xiao K, et al. Biochim Biophys Acta. 2007 Jan;1773(1):8-16. doi: 10.1016/j.bbamcr.2006.07.005. Epub 2006 Jul 21. Biochim Biophys Acta. 2007. PMID: 16949165 Review. - Protecting your tail: regulation of cadherin degradation by p120-catenin.
Kowalczyk AP, Reynolds AB. Kowalczyk AP, et al. Curr Opin Cell Biol. 2004 Oct;16(5):522-7. doi: 10.1016/j.ceb.2004.07.001. Curr Opin Cell Biol. 2004. PMID: 15363802 Review.
Cited by
- Role of N-WASP in Endothelial Monolayer Formation and Integrity.
Mooren OL, Kim J, Li J, Cooper JA. Mooren OL, et al. J Biol Chem. 2015 Jul 24;290(30):18796-805. doi: 10.1074/jbc.M115.668285. Epub 2015 Jun 12. J Biol Chem. 2015. PMID: 26070569 Free PMC article. - Src-induced tyrosine phosphorylation of VE-cadherin is not sufficient to decrease barrier function of endothelial monolayers.
Adam AP, Sharenko AL, Pumiglia K, Vincent PA. Adam AP, et al. J Biol Chem. 2010 Mar 5;285(10):7045-55. doi: 10.1074/jbc.M109.079277. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048167 Free PMC article. - Sphingosine 1-Phosphate Receptor 1 Signaling Maintains Endothelial Cell Barrier Function and Protects Against Immune Complex-Induced Vascular Injury.
Burg N, Swendeman S, Worgall S, Hla T, Salmon JE. Burg N, et al. Arthritis Rheumatol. 2018 Nov;70(11):1879-1889. doi: 10.1002/art.40558. Arthritis Rheumatol. 2018. PMID: 29781582 Free PMC article. - Monocyte Induction of E-Selectin-Mediated Endothelial Activation Releases VE-Cadherin Junctions to Promote Tumor Cell Extravasation in the Metastasis Cascade.
Häuselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grässle S, Bauer AT, Schneider SW, Borsig L. Häuselmann I, et al. Cancer Res. 2016 Sep 15;76(18):5302-12. doi: 10.1158/0008-5472.CAN-16-0784. Epub 2016 Aug 3. Cancer Res. 2016. PMID: 27488527 Free PMC article. - VE-cadherin: at the front, center, and sides of endothelial cell organization and function.
Harris ES, Nelson WJ. Harris ES, et al. Curr Opin Cell Biol. 2010 Oct;22(5):651-8. doi: 10.1016/j.ceb.2010.07.006. Epub 2010 Aug 11. Curr Opin Cell Biol. 2010. PMID: 20708398 Free PMC article. Review.
References
- Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost. 2001;86:308–315. - PubMed
- Gooding JM, Yap KL, Ikura M. The cadherin-catenin complex as a focal point of cell adhesion and signalling: new insights from three-dimensional structures. Bioessays. 2004;26:497–511. - PubMed
- Kowalczyk AP, Navarro P, Dejana E, et al. VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J Cell Sci. 1998;111(pt 20):3045–3057. - PubMed
- Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL036028/HL/NHLBI NIH HHS/United States
- R01 HL032854/HL/NHLBI NIH HHS/United States
- HL32854/HL/NHLBI NIH HHS/United States
- R01 HL053993-13/HL/NHLBI NIH HHS/United States
- P01 HL036028-239002/HL/NHLBI NIH HHS/United States
- R37 HL032854/HL/NHLBI NIH HHS/United States
- R01 HL077870/HL/NHLBI NIH HHS/United States
- R01AR050501/AR/NIAMS NIH HHS/United States
- HL36028/HL/NHLBI NIH HHS/United States
- P01 HL036028-230005/HL/NHLBI NIH HHS/United States
- R01 AR050501/AR/NIAMS NIH HHS/United States
- R01 HL053993/HL/NHLBI NIH HHS/United States
- HL077870/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous