Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets - PubMed (original) (raw)
Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets
Brian Boylan et al. Blood. 2008.
Abstract
Immunoreceptor tyrosine-based activation motif (ITAM)-containing proteins have recently been demonstrated in macrophages and neutrophils to be required for cell surface integrins to transmit activation signals into the cell. To identify ITAM-bearing proteins that mediate signaling via the platelet-specific integrin alphaIIbbeta3, fibrinogen binding was induced by (1) allowing platelets to spread directly on immobilized fibrinogen, or (2) activating the PAR1 thrombin receptor on platelets in suspension. Both initiated strong, ligand binding-dependent tyrosine phosphorylation of the ITAM-bearing platelet Fc receptor, FcgammaRIIa, as well as downstream phosphorylation of the protein tyrosine kinase Syk and activation of phospholipase Cgamma2 (PLCgamma2). Addition of Fab fragments of an FcgammaRIIa-specific monoclonal antibody strongly inhibited platelet spreading on immobilized fibrinogen, as well as downstream tyrosine phosphorylation of FcgammaRIIa, Syk, and PLCgamma2, and platelets from a patient whose platelets express reduced levels of FcgammaRIIa exhibited markedly reduced spreading on immobilized fibrinogen. Finally, fibrinogen binding-induced FcgammaRIIa phosphorylation did not occur in human platelets expressing a truncated beta3 cytoplasmic domain. Taken together, these data suggest that ligand binding to platelet alphaIIbbeta3 induces integrin cytoplasmic domain-dependent phosphorylation of FcgammaRIIa, which then enlists selected components of the immunoreceptor signaling cascade to transmit amplification signals into the cell.
Figures
Figure 1
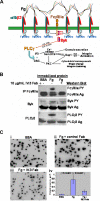
Outside-in signaling initiated by platelet interaction with immobilized fibrinogen activates the FcγRIIa signal transduction pathway. (A) Schematic showing use of the FcγRIIa signaling pathway downstream of αIIbβ3 engagement. Note the importance of physical approximation of the integrin with FcγRIIa, allowing integrin-associated Src family members to function as ITAM kinases, thus initiating the outside-in signaling cascade. (B,C) Washed platelets were plated onto 8-chamber glass tissue-culture slides coated with either BSA or fibrinogen (Fg), and allowed to spread for 45 minutes in the presence or absence of 10 μg/mL IV.3 Fab Immunoprecipitation and Western blot analysis (B) reveals strong activation of FcγRIIa, Syk, and PLCγ2 after platelet binding to immobilized fibrinogen, and inhibition of this outside-in signaling pathway by Fab fragments of mAb IV.3. (C) Effect of IV.3 on platelet spreading. Preincubation of platelets with IV.3 Fab markedly inhibits platelet spreading on immobilized fibrinogen, shown in panels A through C, with quantitative analysis of the pixel area of spread platelets in panel. Data shown are the mean plus or minus SEM from one of 4 representative experiments using 3 different platelet donors.
Figure 2
Agonist-induced activation of human platelets results in tyrosine phosphorylation of FcγRIIa, Syk, and PLCγ2 in a ligand binding–dependent manner. Washed human platelets stirring in an aggregometer cuvette were stimulated with TRAP in the presence or absence of 2 mM RGD peptide or 10 μg/mL IV.3 Fab, as indicated. After detergent lysis, proteins were immunoprecipitated using antibodies specific for FcγRIIa, Syk, or PLCγ2, and immunoblots containing immunoprecipitated proteins stained either for antigen (Ag) or phosphotyrosine (PY). Note that FcγRIIa, Syk, and PLCγ2 each become tyrosine phosphorylated after platelet activation, and that preventing ligand binding with RGD peptide (left half of the figure) largely inhibits tyrosine phosphorylation of each of these signaling components, demonstrating that ligand binding to the integrin αIIbβ3 activates the FcγRIIa→Syk→PLCγ2 signaling pathway. Also note that preincubation of platelets with IV.3 (right half of the figure) prevents FcγRIIa, Syk, and PLCγ2 activation in response to TRAP. Data shown are representative of 3 separate experiments performed using 2 different platelet donors.
Figure 3
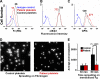
Human platelets expressing decreased FcγRIIa fail to spread on immobilized fibrinogen. (A-C) Flow cytometric analysis of washed platelets from a patient with an autoantibody specific for GPVI, demonstrating approximately 60% reduced expression of FcγRIIa (as reported by the binding if mAb IV.3), but normal expression of αIIbβ3 and the GPIb complex. (D,E) Washed patient platelets or control platelets from a healthy donor were allowed to spread on glass slides that had been coated with 100 μg/mL fibrinogen. The degree of platelet spreading was analyzed at both 30 and 60 minutes. Note that the patient's platelets failed to spread. Data are the mean plus or minus SEM.
Figure 4
The cytoplasmic domain of the integrin β3 subunit is required for ligand binding–induced phosphorylation of FcγRIIa. Washed platelets from a patient expressing a homozygous mutant form of a Δ724 β3 integrin subunit were plated onto immobilized fibrinogen or BSA and allowed to spread for 45 minutes at 37°C. Note the failure of platelets harboring a truncated β3 cytoplasmic tail to support tyrosine phosphorylation of FcγRIIa (A) or to spread on immobilized fibrinogen (B,C). Representative pictures are shown in panel B, with the average pixel area of 148 to 350 platelets per condition quantitated and shown in panel C. Error bars show mean plus or minus SEM.
Similar articles
- Mechanism of outside-in {alpha}IIb{beta}3-mediated activation of human platelets by the colonizing Bacterium, Streptococcus gordonii.
Keane C, Petersen H, Reynolds K, Newman DK, Cox D, Jenkinson HF, Newman PJ, Kerrigan SW. Keane C, et al. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2408-15. doi: 10.1161/ATVBAHA.110.216515. Epub 2010 Nov 11. Arterioscler Thromb Vasc Biol. 2010. PMID: 21071690 Free PMC article. - Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo.
Zhi H, Rauova L, Hayes V, Gao C, Boylan B, Newman DK, McKenzie SE, Cooley BC, Poncz M, Newman PJ. Zhi H, et al. Blood. 2013 Mar 7;121(10):1858-67. doi: 10.1182/blood-2012-07-443325. Epub 2012 Dec 20. Blood. 2013. PMID: 23264598 Free PMC article. - Peptide LSARLAF induces integrin β3 dependent outside-in signaling in platelets.
Niu H, Xu Z, Li D, Zhang L, Wang K, Taylor DB, Liu J, Gartner TK. Niu H, et al. Thromb Res. 2012 Aug;130(2):203-9. doi: 10.1016/j.thromres.2012.03.004. Epub 2012 Apr 5. Thromb Res. 2012. PMID: 22482832 Free PMC article. - Beta3 tyrosine phosphorylation in alphaIIbbeta3 (platelet membrane GP IIb-IIIa) outside-in integrin signaling.
Phillips DR, Nannizzi-Alaimo L, Prasad KS. Phillips DR, et al. Thromb Haemost. 2001 Jul;86(1):246-58. Thromb Haemost. 2001. PMID: 11487013 Review. - Human platelet IgG Fc receptor FcγRIIA in immunity and thrombosis.
Arman M, Krauel K. Arman M, et al. J Thromb Haemost. 2015 Jun;13(6):893-908. doi: 10.1111/jth.12905. Epub 2015 Apr 21. J Thromb Haemost. 2015. PMID: 25900780 Review.
Cited by
- Directly Activating the Integrin αIIbβ3 Initiates Outside-In Signaling by Causing αIIbβ3 Clustering.
Fong KP, Zhu H, Span LM, Moore DT, Yoon K, Tamura R, Yin H, DeGrado WF, Bennett JS. Fong KP, et al. J Biol Chem. 2016 May 27;291(22):11706-16. doi: 10.1074/jbc.M116.716613. Epub 2016 Apr 7. J Biol Chem. 2016. PMID: 27056329 Free PMC article. - Amplification of bacteria-induced platelet activation is triggered by FcγRIIA, integrin αIIbβ3, and platelet factor 4.
Arman M, Krauel K, Tilley DO, Weber C, Cox D, Greinacher A, Kerrigan SW, Watson SP. Arman M, et al. Blood. 2014 May 15;123(20):3166-74. doi: 10.1182/blood-2013-11-540526. Epub 2014 Mar 18. Blood. 2014. PMID: 24642751 Free PMC article. - Signaling during platelet adhesion and activation.
Li Z, Delaney MK, O'Brien KA, Du X. Li Z, et al. Arterioscler Thromb Vasc Biol. 2010 Dec;30(12):2341-9. doi: 10.1161/ATVBAHA.110.207522. Epub 2010 Nov 11. Arterioscler Thromb Vasc Biol. 2010. PMID: 21071698 Free PMC article. Review. - α2β1 integrin, GPVI receptor, and common FcRγ chain on mouse platelets mediate distinct responses to collagen in models of thrombosis.
Marjoram RJ, Li Z, He L, Tollefsen DM, Kunicki TJ, Dickeson SK, Santoro SA, Zutter MM. Marjoram RJ, et al. PLoS One. 2014 Nov 21;9(11):e114035. doi: 10.1371/journal.pone.0114035. eCollection 2014. PLoS One. 2014. PMID: 25415203 Free PMC article. - Key role of integrin α(IIb)β (3) signaling to Syk kinase in tissue factor-induced thrombin generation.
van der Meijden PE, Feijge MA, Swieringa F, Gilio K, Nergiz-Unal R, Hamulyák K, Heemskerk JW. van der Meijden PE, et al. Cell Mol Life Sci. 2012 Oct;69(20):3481-92. doi: 10.1007/s00018-012-1033-2. Epub 2012 Jun 6. Cell Mol Life Sci. 2012. PMID: 22669259 Free PMC article.
References
- Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1:1602–1612. - PubMed
- Abraham RT, Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. - PubMed
- Humphrey MB, Lanier LL, Nakamura MC. Role of ITAM-containing adapter proteins and their receptors in the immune system and bone. Immunol Rev. 2005;208:50–65. - PubMed
- Monroe J. GITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–294. - PubMed
- Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous