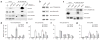The splicing factor SC35 has an active role in transcriptional elongation - PubMed (original) (raw)
The splicing factor SC35 has an active role in transcriptional elongation
Shengrong Lin et al. Nat Struct Mol Biol. 2008 Aug.
Abstract
Mounting evidence suggests that transcription and RNA processing are intimately coupled in vivo, although each process can occur independently in vitro. It is generally thought that polymerase II (Pol II) C-terminal domain (CTD) kinases are recruited near the transcription start site to overcome initial Pol II pausing events, and that stably bound kinases facilitate productive elongation and co-transcriptional RNA processing. Whereas most studies have focused on how RNA processing machineries take advantage of the transcriptional apparatus to efficiently modify nascent RNA, here we report that a well-studied splicing factor, SC35, affects transcriptional elongation in a gene-specific manner. SC35 depletion induces Pol II accumulation within the gene body and attenuated elongation, which are correlated with defective P-TEFb (a complex composed of CycT1-CDK9) recruitment and dramatically reduced CTD Ser2 phosphorylation. Recombinant SC35 is sufficient to rescue this defect in nuclear run-on experiments. These findings suggest a reciprocal functional relationship between the transcription and splicing machineries during gene expression.
Figures
Figure 1
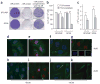
SR proteins are required for Pol II transcription in MEFs. (a) SR proteins are essential for viability of MEFs. Cells were stained with crystal violet 10 d after mock depletion (+SR protein) and Dox-induced SR protein depletion (−SR protein). HA, hemagluttinin. (b) Reduction of nascent transcripts in SR protein–depleted cells (5 d after Dox treatment), as determined by 3H labeling. Error bars indicate s.d. from three independent experiments, and statistical significance in each pairwise comparison is indicated (*P < 0.05; **P < 0.01). (c) Reduction of 3H-labeled Poly(A)+ mRNA. Equal amounts of purified Poly(A)+ mRNA were counted. Error bars indicate s.d. from three independent experiments, and statistical significance in each pairwise comparison is indicated (*P < 0.05; **P < 0.01). (d–k) Selective inhibition of transcription in the nucleoplasm of SC35-depleted MEFs. Mock-depleted (+SC35) and SC35-depleted (−SC35) cells were double stained with anti-BrU antibody for nascent RNA (red) or DAPI (blue) in combination with phalloidin for cytoplasmic actin (green). The remaining BrU signals in the nuclei of SC35-depleted cells colocalized with the nucleolar marker Nucleophosmin (NPM), indicating they are due to Pol I transcription (inset in g: blue, DAPI staining of a nucleus; red, anti-BrU staining of nascent RNA; green, anti-NPM staining of the nucleolus). In the presence of a low dose of ActD, Pol I transcription was selectively suppressed in nucleoli, allowing better visualization of SC35 depletion–induced reduction of Pol II transcription in the nucleoplasm (i,k).
Figure 2
Induced Pol II accumulation in gene bodies in response to SC35 depletion in vivo. Two representative genes (Ptb1 and Ets1) in which Pol II was found to accumulate in specific regions of the gene body detected on a tiling array (a,b), and a representative gene (Ptb2) in which Pol II profiling was unaffected by SR protein depletion (c). Above, Pol II tiling array profiling on the representative genes in mock-depleted (+SC35 or +SF2/ASF) and SR protein–depleted (−SC35 or −SF2/ASF) MEFs. Arrows indicate accumulated Pol II on gene bodies. Gene structures and the locations of primer sets for qPCR validation are indicated underneath the profiles. Below, validation by ChIP and qPCR. Primer sets targeting an upstream intergenic region (InG, see the primer sequence in Supplementary Fig. 3); the beginning, middle and end of each gene were used to quantify immunoprecipitated DNA by qPCR. Data were normalized against the signal on InG. Error bars indicate s.d. based on three independent experiments, and statistical significance in each pairwise comparison is indicated (**P < 0.01).
Figure 3
Requirement for SC35 in transcription elongation. (a) Total RNA extracted from mock-depleted (+SC35 or +SF2/ASF) and SR protein–depleted (−SC35 or −SF2/ASF) cells was analyzed by RT-qPCR using the indicated primer sets, which target different intronic locations in the Ptb1 gene. The GAPDH mRNA level was unaffected and served as a control. No signal was detected if the reverse transcription step was omitted (not shown). All data from mock-depleted cells were normalized to 1.0 and the ratio reflects the fold change of the RNA from SC35-depleted cells over mock-depleted cells. (b) Diagram for the nuclear run-on assay based on the use of BrUTP. The specificity of BrU-labeled nascent Ptb1 transcript detection was demonstrated by analyzing total RNA from wild-type MEFs labeled with BrU, uridine (U) or BrU in the presence of ActD. (c) Nuclear run-on assay on the nascent Ptb1 transcript. The gene structure and the probe positions (A to K) are indicated on the top. Note that the four probes against Ptb1 used in a (p1 to p4) are included in this expanded probe set to achieve fine mapping of transcription attenuation in the nuclear run-on assay. A representative set of RT-PCR data are shown. RT-PCR products from mock-depleted (+SC35) and SC35-depleted (−SC35) MEFs are indicated below each set. No signal was detected without reverse transcription in these reactions. (d) RT-qPCR analysis of the nuclear run-on assay as in c. U2 small nuclear RNA (snRNA) was unaffected by SC35 depletion, which was assayed as a control. Error bars indicate s.d. based on three independent experiments.
Figure 4
Functional rescue of SC35 depletion–induced blockage of transcriptional elongation. (a) Reconstitution of nuclear run-on using recombinant SC35. Each set of three PCR products represents the analysis of RNA from mock-depleted MEFs (+endogenous (endo) SC35), SC35-depleted MEFs (−endo SC35) and SC35-depleted MEFs plus recombinant SC35 (rSC35). (b) RT-qPCR analysis of the nuclear run-on assay as in a. U2 snRNA was unaffected by SC35 depletion, which was assayed as a control. Error bars indicate s.d. based on three independent repeats of each reaction. (c) Addition of recombinant SC35 did not generally stimulate transcriptional elongation in mock-depleted cells. Error bars indicate s.d. based on three independent repeats of the experiment using a subset of radioactive PCR primers used in a. qPCR analysis based on the use of the entire primer set gave rise to the same result (data not shown). (d) Specificity in the reconstituted nuclear run-on assay. SC35-depleted MEFs (−endo SC35) treated with digitonin were supplemented with recombinant 9G8 and SF2/ASF. This experiment was carried out alongside the experiment in a, but using a subset of radioactive PCR primers as indicated.
Figure 5
Dynamic and SC35-dependent recruitment of P-TEFb to the elongating Pol II complex. (a) Co-immunoprecipitation of hemagglutinin (HA)-tagged SC35 with Pol II and key factors (CDK9, the kinase component of P-TEFb and TAT-SF1) involved in transcriptional elongation. (b) The phosphorylation state of Pol II in mock-depleted and SC35-depleted MEFs. Specific antibodies used in western blotting are indicated on the right. (c) Impact of SC35 depletion on P-TEFb association with Pol II. Immunoprecipitation (IP) with anti–Pol II was carried out in mock-depleted and SC35-depleted MEFs followed by western blotting using anti-CDK9. (d–g) ChIP and qPCR analysis of the Pol II phosphorylation state and CDK9 binding in the Ptb1 gene. The data were normalized against the background signal from an intergenic region (InG) upstream of the Ptb1 promoter. Error bars indicate s.d. based on three independent experiments.
Figure 6
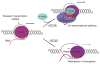
Proposed model for the role of SR proteins in transcriptional elongation and co-transcriptional RNA splicing. During transcription, a transient bubble forms, which collapses quickly during transcriptional elongation. In wild-type cells (above right), SC35 and SC35-recruited factors may travel with the Pol II complex to scan emerging _cis_-acting RNA elements in nascent transcripts, which may contribute to the displacement of RNA from template DNA. Such initial scanning may help nucleate the assembly of the spliceosome on nascent transcripts after additional cooperative _cis_-acting splicing signals become available. In the absence of SC35 (below right), dynamic P-TEFb recruitment and Pol II CTD Ser2 phosphorylation are inefficient or impaired, thereby retarding the elongation of the Pol II complex in general and/or at certain locations within gene bodies.
Comment in
- A splicing regulator promotes transcriptional elongation.
Fededa JP, Kornblihtt AR. Fededa JP, et al. Nat Struct Mol Biol. 2008 Aug;15(8):779-81. doi: 10.1038/nsmb0808-779. Nat Struct Mol Biol. 2008. PMID: 18679431 No abstract available.
Similar articles
- A human splicing factor, SKIP, associates with P-TEFb and enhances transcription elongation by HIV-1 Tat.
Brès V, Gomes N, Pickle L, Jones KA. Brès V, et al. Genes Dev. 2005 May 15;19(10):1211-26. doi: 10.1101/gad.1291705. Genes Dev. 2005. PMID: 15905409 Free PMC article. - RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II.
Yu M, Yang W, Ni T, Tang Z, Nakadai T, Zhu J, Roeder RG. Yu M, et al. Science. 2015 Dec 11;350(6266):1383-6. doi: 10.1126/science.aad2338. Science. 2015. PMID: 26659056 Free PMC article. - SR proteins collaborate with 7SK and promoter-associated nascent RNA to release paused polymerase.
Ji X, Zhou Y, Pandit S, Huang J, Li H, Lin CY, Xiao R, Burge CB, Fu XD. Ji X, et al. Cell. 2013 May 9;153(4):855-68. doi: 10.1016/j.cell.2013.04.028. Cell. 2013. PMID: 23663783 Free PMC article. - P-TEFb stimulates transcription elongation and pre-mRNA splicing through multilateral mechanisms.
Lenasi T, Barboric M. Lenasi T, et al. RNA Biol. 2010 Mar-Apr;7(2):145-50. doi: 10.4161/rna.7.2.11057. Epub 2010 Mar 29. RNA Biol. 2010. PMID: 20305375 Review. - The multi-tasking P-TEFb complex.
Brès V, Yoh SM, Jones KA. Brès V, et al. Curr Opin Cell Biol. 2008 Jun;20(3):334-40. doi: 10.1016/j.ceb.2008.04.008. Epub 2008 May 29. Curr Opin Cell Biol. 2008. PMID: 18513937 Free PMC article. Review.
Cited by
- RSR-2, the Caenorhabditis elegans ortholog of human spliceosomal component SRm300/SRRM2, regulates development by influencing the transcriptional machinery.
Fontrodona L, Porta-de-la-Riva M, Morán T, Niu W, Díaz M, Aristizábal-Corrales D, Villanueva A, Schwartz S Jr, Reinke V, Cerón J. Fontrodona L, et al. PLoS Genet. 2013 Jun;9(6):e1003543. doi: 10.1371/journal.pgen.1003543. Epub 2013 Jun 6. PLoS Genet. 2013. PMID: 23754964 Free PMC article. - Transcription factories.
Rieder D, Trajanoski Z, McNally JG. Rieder D, et al. Front Genet. 2012 Oct 23;3:221. doi: 10.3389/fgene.2012.00221. eCollection 2012. Front Genet. 2012. PMID: 23109938 Free PMC article. - The function of introns.
Chorev M, Carmel L. Chorev M, et al. Front Genet. 2012 Apr 13;3:55. doi: 10.3389/fgene.2012.00055. eCollection 2012. Front Genet. 2012. PMID: 22518112 Free PMC article. - RNA polymerase II elongation control.
Zhou Q, Li T, Price DH. Zhou Q, et al. Annu Rev Biochem. 2012;81:119-43. doi: 10.1146/annurev-biochem-052610-095910. Epub 2012 Mar 9. Annu Rev Biochem. 2012. PMID: 22404626 Free PMC article. Review. - Biologic and clinical significance of somatic mutations of SF3B1 in myeloid and lymphoid neoplasms.
Cazzola M, Rossi M, Malcovati L; Associazione Italiana per la Ricerca sul Cancro Gruppo Italiano Malattie Mieloproliferative. Cazzola M, et al. Blood. 2013 Jan 10;121(2):260-9. doi: 10.1182/blood-2012-09-399725. Epub 2012 Nov 16. Blood. 2013. PMID: 23160465 Free PMC article. Review.
References
- Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. - PubMed
- Lacadie SA, Rosbash M. Cotranscriptional spliceosome assembly dynamics and the role of U1 snRNA:5′ ss base pairing in yeast. Mol Cell. 2005;19:65–75. - PubMed
- Gornemann J, Kotovic KM, Hujer K, Neugebauer KM. Cotranscriptional spliceosome assembly occurs in a stepwise fashion and requires the cap binding complex. Mol Cell. 2005;19:53–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5R01 GM49369/GM/NIGMS NIH HHS/United States
- R01 GM049369-08/GM/NIGMS NIH HHS/United States
- R01 GM049369-15/GM/NIGMS NIH HHS/United States
- F32 GM077907/GM/NIGMS NIH HHS/United States
- F32 GM077907-02/GM/NIGMS NIH HHS/United States
- 1F32 GM077907/GM/NIGMS NIH HHS/United States
- R01 GM049369/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous