Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy - PubMed (original) (raw)
Hsp70 overexpression inhibits NF-kappaB and Foxo3a transcriptional activities and prevents skeletal muscle atrophy
Sarah M Senf et al. FASEB J. 2008 Nov.
Abstract
Heat shock protein 70 (Hsp70) is a highly conserved and ubiquitous protein that is reported to provide cytoprotection in various cell types and tissues. However, the importance of Hsp70 expression during skeletal muscle atrophy, when Hsp70 levels are significantly decreased, is not known. The current study aimed to determine whether plasmid-mediated overexpression of Hsp70, in the soleus muscle of rats, was sufficient to regulate specific atrophy signaling pathways and attenuate skeletal muscle disuse atrophy. We found that Hsp70 overexpression prevented disuse muscle fiber atrophy and inhibited the increased promoter activities of atrogin-1 and MuRF1. Importantly, the transcriptional activities of Foxo3a and NF-kappaB, which are implicated in the regulation of atrogin-1 and MuRF1, were abolished by Hsp70. These data suggest that Hsp70 may regulate key atrophy genes through inhibiting Foxo3a and NF-kappaB activities during disuse. Indeed, we show that specific inhibition of Foxo3a prevented the increases in both atrogin-1 and MuRF1 promoter activities during disuse. However, inhibition of NF-kappaB did not affect the activation of either promoter, suggesting its requirement for disuse atrophy is through its regulation of other atrophy genes. We conclude that overexpression of Hsp70 is sufficient to inhibit key atrophy signaling pathways and prevent skeletal muscle atrophy.
Figures
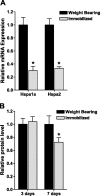
Figure 1.
Hind-limb immobilization decreases Hsp70 mRNA and protein levels. A) Hspa1a and Hspa2 mRNA expression from weight-bearing and immobilized soleus muscles after 3 days. B) Hsp70 protein expression from weight-bearing and immobilized soleus muscles after 3 days and 7 days. Bars represent means ±
se
from 6 muscles. *P < 0.05 vs. weight bearing.
Figure 2.
Overexpression of Hsp70. A) Representative Western blot of whole-cell lysates from solei injected with either a control or Hsp70 expression plasmid and blotted for Hsp70. B) Quantification of Hsp70 levels from muscle extracts described in A. C) Representative Western blot of whole-cell lysates from solei injected with either EGFP or Hsp70-EGFP and blotted for Hsp70. EGFP is 27 kDa; therefore, Hsp70-EGFP is ∼97 kDa. D) Quantification of Hsp70 levels from muscle extracts described in C. Bars represent means ±
se
from ≥6 muscles. *P < 0.05 vs. control.
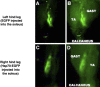
Figure 3.
Localization of the overexpressed Hsp70-EGFP to the soleus muscle. Representative in vivo images (MousePOD; LI-COR) of a left and right rat hind leg injected with EGFP or Hsp70-EGFP, respectively. A, C) Brightness low to demonstrate localization of fluorescent signal to one area. B, D) Brightness increased to show the outline of the hind leg and, therefore, to identify the soleus as the muscle of localized fluorescence. TA, tibialis anterior; GAST, gastrocnemius.
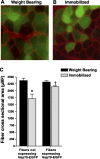
Figure 4.
Hsp70 overexpression prevents skeletal muscle fiber atrophy. A, B) Representative cross sections taken from the soleus muscle of weight bearing (A) and 7 day immobilized rats (B) injected with Hsp70-EGFP. The cross-sectional area of green fluorescent fibers (fibers expressing Hsp70-EGFP) were compared to the cross-sectional area of nonfluorescent fibers (fibers not expressing Hsp70-EGFP) within the same muscle. C) Muscle fiber cross-sectional area of ∼250 fibers/muscle, from 6 muscles/group. *P < 0.05 vs. nonexpressing weight-bearing fibers; †P < 0.05 vs. nonexpressing immobilized fibers.
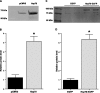
Figure 5.
Hsp70 inhibits the immobilization-induced increase in ubiquitin ligases. A) mRNA expression of MAFbx/atrogin-1, MuRF1, and Nedd4 in weight-bearing and 3 day immobilized soleus muscles injected with either a control or Hsp70 plasmid. The standardization of loading is illustrated by the unchanged 18S rRNA. B, C) Atrogin-1 (B) and MuRF1 (C) promoter reporter activity from the soleus muscles of weight-bearing and 3-day immobilized rats, injected with a control or Hsp70 plasmid plus the respective promoter reporter plasmid. D) Total ubiquitinated proteins from weight-bearing and 7 day immobilized soleus muscles injected with either a control or Hsp70 plasmid. E) Representative Western blot of whole-cell lysates from weight-bearing or immobilized muscles injected with either a control or Hsp70 expression plasmid and blotted for ubiquitin. The sum fluorescence of each lane was used to quantify total ubiquitinated protein of that sample. Bars represent means ±
se
from ≥6 muscles. *P < 0.05 vs. weight-bearing control; †P < 0.05 vs. immobilized control.
Figure 6.
Hsp70 abolishes immobilization-induced NF-κB and FOXO3a transcriptional activation. NF-κB (A) and Foxo3a (B) reporter activity from the soleus muscle of weight-bearing and 3 and 7 day immobilized rats, injected with either a control or Hsp70 plasmid plus the respective reporter plasmid. Bars represent means ±
se
from 6 or 8 muscles (3 and 7 day study, respectively). *P < 0.05 vs. weight-bearing control.
Figure 7.
Foxo3a, but not NF-κB, is required for the immobilization-induced increases in Atrogin-1 and MuRF1 promoter activities. A) NF-κB reporter activity from the soleus muscle of weight bearing and 3-day immobilized rats injected with either a control plasmid or the IκBα super repressor (IκBα SR). B) Foxo3a reporter activity from the soleus muscle of weight bearing and 3-day immobilized rats injected with a control plasmid or d.n.Foxo3a. C, D) Atrogin-1 (C) and MuRF1 (D) promoter reporter activity from the soleus muscles of weight bearing or 3-day immobilized rats injected with a control plasmid, the IκBα super repressor, or d.n.Foxo3a. Bars represent means ±
se
from 6 muscles. *P < 0.05 vs. weight-bearing control; †P < 0.05 vs. immobilized control.
Similar articles
- Hsp27 inhibits IKKbeta-induced NF-kappaB activity and skeletal muscle atrophy.
Dodd SL, Hain B, Senf SM, Judge AR. Dodd SL, et al. FASEB J. 2009 Oct;23(10):3415-23. doi: 10.1096/fj.08-124602. Epub 2009 Jun 15. FASEB J. 2009. PMID: 19528257 Free PMC article. - FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70.
Senf SM, Dodd SL, Judge AR. Senf SM, et al. Am J Physiol Cell Physiol. 2010 Jan;298(1):C38-45. doi: 10.1152/ajpcell.00315.2009. Epub 2009 Oct 28. Am J Physiol Cell Physiol. 2010. PMID: 19864323 Free PMC article. - Muscle-specific microRNA1 (miR1) targets heat shock protein 70 (HSP70) during dexamethasone-mediated atrophy.
Kukreti H, Amuthavalli K, Harikumar A, Sathiyamoorthy S, Feng PZ, Anantharaj R, Tan SL, Lokireddy S, Bonala S, Sriram S, McFarlane C, Kambadur R, Sharma M. Kukreti H, et al. J Biol Chem. 2013 Mar 1;288(9):6663-78. doi: 10.1074/jbc.M112.390369. Epub 2013 Jan 6. J Biol Chem. 2013. PMID: 23297411 Free PMC article. - Skeletal muscle hypertrophy and atrophy signaling pathways.
Glass DJ. Glass DJ. Int J Biochem Cell Biol. 2005 Oct;37(10):1974-84. doi: 10.1016/j.biocel.2005.04.018. Int J Biochem Cell Biol. 2005. PMID: 16087388 Review. - The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy.
Foletta VC, White LJ, Larsen AE, Léger B, Russell AP. Foletta VC, et al. Pflugers Arch. 2011 Mar;461(3):325-35. doi: 10.1007/s00424-010-0919-9. Epub 2011 Jan 11. Pflugers Arch. 2011. PMID: 21221630 Review.
Cited by
- Loss of the inducible Hsp70 delays the inflammatory response to skeletal muscle injury and severely impairs muscle regeneration.
Senf SM, Howard TM, Ahn B, Ferreira LF, Judge AR. Senf SM, et al. PLoS One. 2013 Apr 23;8(4):e62687. doi: 10.1371/journal.pone.0062687. Print 2013. PLoS One. 2013. PMID: 23626847 Free PMC article. - Identification of a conserved set of upregulated genes in mouse skeletal muscle hypertrophy and regrowth.
Chaillou T, Jackson JR, England JH, Kirby TJ, Richards-White J, Esser KA, Dupont-Versteegden EE, McCarthy JJ. Chaillou T, et al. J Appl Physiol (1985). 2015 Jan 1;118(1):86-97. doi: 10.1152/japplphysiol.00351.2014. Epub 2014 Nov 13. J Appl Physiol (1985). 2015. PMID: 25554798 Free PMC article. - Post-exercise Cold Water Immersion Effects on Physiological Adaptations to Resistance Training and the Underlying Mechanisms in Skeletal Muscle: A Narrative Review.
Petersen AC, Fyfe JJ. Petersen AC, et al. Front Sports Act Living. 2021 Apr 8;3:660291. doi: 10.3389/fspor.2021.660291. eCollection 2021. Front Sports Act Living. 2021. PMID: 33898988 Free PMC article. Review. - CORP: Gene delivery into murine skeletal muscle using in vivo electroporation.
Hughes DC, Hardee JP, Waddell DS, Goodman CA. Hughes DC, et al. J Appl Physiol (1985). 2022 Jul 1;133(1):41-59. doi: 10.1152/japplphysiol.00088.2022. Epub 2022 May 5. J Appl Physiol (1985). 2022. PMID: 35511722 Free PMC article. Review. - Transcriptional adaptations following exercise in thoroughbred horse skeletal muscle highlights molecular mechanisms that lead to muscle hypertrophy.
McGivney BA, Eivers SS, MacHugh DE, MacLeod JN, O'Gorman GM, Park SD, Katz LM, Hill EW. McGivney BA, et al. BMC Genomics. 2009 Dec 30;10:638. doi: 10.1186/1471-2164-10-638. BMC Genomics. 2009. PMID: 20042072 Free PMC article.
References
- Liu, Y., Gampert, L., Nething, K., Steinacker, J. M. (2006) Response and function of skeletal muscle heat shock protein 70. Front. Biosci. ,2802-2827 - PubMed
- Selsby, J. T., Rother, S., Tsuda, S., Pracash, O., Quindry, J., Dodd, S. L. (2007) Intermittent hyperthermia enhances skeletal muscle regrowth and attenuates oxidative damage following reloading. J. Appl. Physiol. ,1702-1707 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials