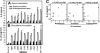Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kappaB - PubMed (original) (raw)
Campylobacter-induced interleukin-8 secretion in polarized human intestinal epithelial cells requires Campylobacter-secreted cytolethal distending toxin- and Toll-like receptor-mediated activation of NF-kappaB
Jie Zheng et al. Infect Immun. 2008 Oct.
Abstract
Campylobacter jejuni and Campylobacter coli colonize and infect the intestinal epithelium and cause acute inflammatory diarrhea. The intestinal epithelium serves as a physical barrier to, and a sensor of, bacterial infection by secreting proinflammatory cytokines. This study examined the mechanisms for Campylobacter-induced secretion of the proinflammatory chemokine interleukin-8 (IL-8) by using polarized T84 human colonic epithelial cells as a model. C. jejuni increased the secretion of both IL-8 and tumor necrosis factor alpha (TNF-alpha) in polarized epithelial cells. However, the increase in IL-8 secretion was independent of Campylobacter-stimulated TNF-alpha secretion. Polarized T84 cells secreted IL-8 predominantly to the basolateral medium independently of the inoculation direction. While there was a significant correlation between the levels of IL-8 secretion and Campylobacter invasion, all 11 strains tested increased IL-8 secretion by polarized T84 cells despite their differences in adherence, invasion, and transcytosis efficiencies. Cell-free supernatants of Campylobacter-T84-cell culture increased IL-8 secretion to levels similar to those induced by live bacterial inoculation. The ability of the supernatant to induce IL-8 secretion was reduced by flagellum and cytolethal distending toxin (CDT) gene mutants, treatment of the supernatant with protease K or heat, or treatment of T84 cells with the Toll-like receptor (TLR) inhibitor MyD88 inhibitory peptide or chloroquine. NF-kappaB inhibitors or cdtB mutation plus MyD88 inhibitor, but not flaA cdtB double mutations, abolished the ability of the supernatant to induce IL-8 secretion. Taken together, our results demonstrate that Campylobacter-induced IL-8 secretion requires functional flagella and CDT and depends on the activation of NF-kappaB through TLR signaling and CDT in human intestinal epithelial cells.
Figures
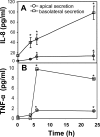
FIG. 1.
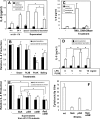
Campylobacter inoculation increased the secretion of IL-8 and TNF-α in polarized human intestinal epithelial T84 cells. T84 cells were cultured on transwells until the transepithelial resistance reached 1,400 Ω/cm2. C. jejuni 81-176 was inoculated from the apical chamber at an MOI of ∼10 and incubated at 37°C for 0, 4, 6, or 24 h. For the 24-h time point, the cells were washed at 6 h to remove unattached bacteria and cultured in fresh medium for another 18 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation at any time point. The supernatants from the apical and basolateral chambers were collected separately; the bacteria were removed by centrifugation; and protease inhibitors were added to prevent protein degradation. The concentrations of IL-8 (A) and TNF-α (B) were determined by ELISA. Averages for three independent experiments are shown; error bars, standard deviations. Asterisks represent significant (P < 0.05) differences from control cells that were not inoculated with the bacteria.
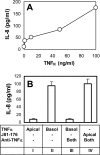
FIG. 2.
_Campylobacter_-stimulated IL-8 secretion is not dependent on the secretion of TNF-α. (A) Polarized T84 cells were incubated with different concentrations of TNF-α (0, 5, 10, 50, and 100 ng/ml) basolaterally at 37°C for 24 h. The basolateral media were collected, and the concentrations of IL-8 were measured using ELISA. (B) IL-8 concentrations in the basolateral medium were measured using ELISA at 24 h after T84 cells were incubated under the following conditions: TNF-α (50 ng/ml) applied apically (Apical) (bar I), TNF-α applied basolaterally (Basol) (bar II), (III) TNF-α applied basolaterally plus mouse anti-human TNF-α antibody (5 μg/ml) applied both apically and basolaterally (Both) (bar III), or live C. jejuni 81-176 applied apically plus mouse anti-human TNF-α antibody applied both apically and basolaterally (bar IV). Averages from three independent experiments are shown; error bars, standard deviations.
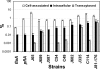
FIG. 3.
Adherence, invasion, and transcytosis abilities of different Campylobacter strains in polarized human intestinal epithelial cells. Polarized T84 cells were inoculated with different chicken isolates (C. coli [C] or C. jejuni [J] strains) or with human clinical isolate C. jejuni 81-176 or its flaA or pflA mutant from the apical chambers of transwells at an MOI of ∼10 and were incubated at 37°C for 4 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. The medium from the basolateral chamber was collected to determine the number of transcytosed bacteria. T84 cells were washed and lysed to determine the number of host cell-associated bacteria. Parallel transwells were treated with 100 μg/ml gentamicin, washed, and lysed to determine the number of intracellular bacteria. The data are presented as percentages of the inocula and are averages for three independent experiments with triplicate samples. Error bars, standard deviations.
FIG. 4.
Comparison of IL-8 secretion induced by different Campylobacter strains inoculated apically or basolaterally. (A and B) Polarized T84 cells were inoculated from either the apical (A) or the basolateral (B) side with chicken meat isolates (C. coli [C] or C. jejuni [J] strains) or with C. jejuni 81-176 or its flaA mutant at an MOI of ∼10 and were incubated for 24 h. At 6 h, T84 cells were washed and placed in fresh medium. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. Uninfected T84 cells were used as controls. The apical and basolateral media were collected separately at 24 h, and the concentrations of IL-8 were measured by ELISA. Averages for three independent experiments are shown; error bars, standard deviations. (C) Pearson's correlation coefficients between IL-8 secretion and Campylobacter adherence, invasion, and transcytosis efficiencies (expressed as percentages of the inocula) were calculated and tested for significance (α = 0.05 by a two-tailed test).
FIG. 5.
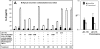
IL-8 secretion induced by conditioned supernatants generated from _Campylobacter_-T84 cell coculture. (A) Polarized T84 cells were incubated for 24 h either with bacterium-free conditioned supernatants generated from the apical or basolateral supernatant of polarized T84 cells that had been inoculated with C. jejuni 81-176 for 4 h or with C. jejuni 81-176 alone cultured in the invasion medium for 4 h (bacterial). T84 cells were incubated with the bacterial culture medium in the apical chamber, with the apical conditioned supernatant in the apical chamber, or with the basolateral conditioned supernatant in the basolateral chamber. Polarized T84 cells inoculated apically with live C. jejuni 81-176 for 4 h and 24 h were used as controls. (B) The basolateral conditioned supernatant generated from C. jejuni 81-176-T84 cell coculture either was not pretreated (−) or was pretreated with either DNase I (10 U/ml) at 37°C for 2 h, polymyxin B (20 μg/ml) at 37°C for 30 min (PLXB), protease K (100 μg/ml) overnight followed by a 20-min incubation at 100°C (ProtK), or a 20-min incubation at 100°C without protease K (Boiling). Polarized T84 cells were incubated with the pretreated or untreated conditioned supernatants from wt C. jejuni 81-176-T84 cell coculture in the basolateral chamber at 37°C for 24 h. (C) Polarized T84 cells were treated basolaterally with a C. jejuni 81-176 DNA extract (25 μg/ml) (DNA) and DNase I-treated C. jejuni 81-176 DNA (DNA + DNase) at 37°C for 24 h. (D) Polarized T84 cells were incubated basolaterally with different concentrations of E. coli LPS in the presence or absence of 20 μg/ml PLXB. (E) Polarized T84 cells were incubated with conditioned supernatants from a coculture of T84 cells with wt C. jejuni 81-176 or its flaA, pflA, cdtB, or flaA cdtB mutant in the basolateral chamber at 37°C for 24 h. After different treatments, the apical (filled bars) and basolateral (open bars) media were collected. IL-8 concentrations were determined by ELISA. The data are means for three independent experiments; error bars, standard deviations. The data in panels B and E are expressed as the ratio of the IL-8 level induced by a treated basolateral conditioned supernatant to that induced by an untreated supernatant and as the ratio of the IL-8 level induced by a mutant basolateral conditioned supernatant to that induced by a wt supernatant. *, P < 0.05. (F) The cytotoxicity of CDT was measured by incubating Vero cells with serially diluted C. jejuni culture supernatants that were filtrated and treated with polymyxin B for 48 h. The viability of the Vero cells was monitored using an MTT assay. The CDT titers were expressed as the reciprocal of the highest dilution that caused 50% Vero cell death compared with the level in untreated cells. Data are averages for three independent cytotoxicity assays; error bars, standard deviations.
FIG. 6.
_Campylobacter_-induced IL-8 secretion depends on _Campylobacter_-secreted CDT and the TLR signaling adaptor MyD88 in polarized intestinal epithelial cells. (A) Polarized T84 cells were pretreated with MyD88 inhibitory peptide (100 μM) (bars III, V, and VII) or its control peptide (bars II, IV, and VI) for 24 h or with chloroquine (5 μM) (bars VIII, IX, and XIII) for 30 min before being incubated with the conditioned supernatant (ConSup) from the coculture of T84 cells with wt C. jejuni 81-176 (bars II, III, and VIII), its cdtB mutant (bars IV, V, and IX), or a C. jejuni 81-176 DNA extract (25 μg/ml) (bars VI and VII) at 37°C for 24 h. MyD88 inhibitory peptide, its control peptide, or chloroquine was also included during the 24-h incubation. Polarized T84 cells incubated with TNF-α only (bar XII) or TNF-α plus chloroquine (bar XIII) were used as controls for the effect of chloroquine on IL-8 secretion. Polarized T84 cells incubated with the medium alone for 24 h (bars I) or with live C. jejuni 81-176 for 4 h (bars X), after which the conditioned supernatants were collected, or 24 h (bars XI) were used as controls. In the 24-h incubation with live bacteria, unbound bacteria were removed at 6 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. The apical and basolateral media were collected individually, and the IL-8 concentrations were determined by ELISA. The data are means for three independent experiments; error bars, standard deviations. (B) Polarized T84 cells were pretreated with chloroquine (5 μM) for 30 min before incubation with C. jejuni 81-176 (MOI, ∼10) at 37°C for 4 h. The abilities of C. jejuni 81-176 to adhere to, invade, and transcytose across chloroquine-treated T84 cells were analyzed as described in the legend to Fig. 3. Averages for three independent experiments with triplicate samples are shown; error bars, standard deviations.
FIG. 7.
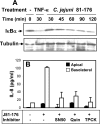
_Campylobacter_-induced IL-8 secretion is dependent on NF-κB activation. (A) T84 cells were incubated with C. jejuni 81-176 (MOI, ∼10) in the presence of cycloheximide for varying lengths of time. T84 cells treated with TNF-α were used as a positive control. −, no treatment. The cells were washed and lysed, and the cell lysates were analyzed by SDS-PAGE and Western blotting with probing for IκB-α. The blots were stripped and reprobed for tubulin as a loading control. Representative results from three independent experiments are shown. (B) Polarized T84 cells were pretreated with the NF-κB inhibitor SN50 (18 μM), quinazoline (28 μM), or TPCK (50 μM) for 30 min and then incubated with C. jejuni 81-176 in the presence of the inhibitor at 37°C for 24 h. No significant difference was detected between the transepithelial resistance before incubation and that after incubation. The apical and basolateral media were collected individually, and the IL-8 concentrations were determined by ELISA. The data are means for three independent experiments; error bars, standard deviations.
Similar articles
- Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells.
Hickey TE, McVeigh AL, Scott DA, Michielutti RE, Bixby A, Carroll SA, Bourgeois AL, Guerry P. Hickey TE, et al. Infect Immun. 2000 Dec;68(12):6535-41. doi: 10.1128/IAI.68.12.6535-6541.2000. Infect Immun. 2000. PMID: 11083762 Free PMC article. - Campylobacter jejuni: targeting host cells, adhesion, invasion, and survival.
Kemper L, Hensel A. Kemper L, et al. Appl Microbiol Biotechnol. 2023 May;107(9):2725-2754. doi: 10.1007/s00253-023-12456-w. Epub 2023 Mar 21. Appl Microbiol Biotechnol. 2023. PMID: 36941439 Free PMC article. Review.
Cited by
- Reduced intestinal epithelial mitochondrial function enhances in vitro interleukin-8 production in response to commensal Escherichia coli.
Saxena A, Lopes F, McKay DM. Saxena A, et al. Inflamm Res. 2018 Oct;67(10):829-837. doi: 10.1007/s00011-018-1172-5. Epub 2018 Jul 20. Inflamm Res. 2018. PMID: 30030553 - Molecular Mechanisms and Potential Clinical Applications of Campylobacter jejuni Cytolethal Distending Toxin.
Lai CK, Chen YA, Lin CJ, Lin HJ, Kao MC, Huang MZ, Lin YH, Chiang-Ni C, Chen CJ, Lo UG, Lin LC, Lin H, Hsieh JT, Lai CH. Lai CK, et al. Front Cell Infect Microbiol. 2016 Feb 9;6:9. doi: 10.3389/fcimb.2016.00009. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 26904508 Free PMC article. Review. - Worm burden-dependent disruption of the porcine colon microbiota by Trichuris suis infection.
Wu S, Li RW, Li W, Beshah E, Dawson HD, Urban JF Jr. Wu S, et al. PLoS One. 2012;7(4):e35470. doi: 10.1371/journal.pone.0035470. Epub 2012 Apr 20. PLoS One. 2012. PMID: 22532855 Free PMC article. - Modification of intestinal microbiota and its consequences for innate immune response in the pathogenesis of campylobacteriosis.
Masanta WO, Heimesaat MM, Bereswill S, Tareen AM, Lugert R, Groß U, Zautner AE. Masanta WO, et al. Clin Dev Immunol. 2013;2013:526860. doi: 10.1155/2013/526860. Epub 2013 Nov 14. Clin Dev Immunol. 2013. PMID: 24324507 Free PMC article. Review. - Campylobacter jejuni translocation across intestinal epithelial cells is facilitated by ganglioside-like lipooligosaccharide structures.
Louwen R, Nieuwenhuis EE, van Marrewijk L, Horst-Kreft D, de Ruiter L, Heikema AP, van Wamel WJ, Wagenaar JA, Endtz HP, Samsom J, van Baarlen P, Akhmanova A, van Belkum A. Louwen R, et al. Infect Immun. 2012 Sep;80(9):3307-18. doi: 10.1128/IAI.06270-11. Epub 2012 Jul 9. Infect Immun. 2012. PMID: 22778098 Free PMC article.
References
- Abreu, M. T., M. Fukata, and M. Arditi. 2005. TLR signaling in the gut in health and disease. J. Immunol. 1744453-4460. - PubMed
- Abreu, M. T., P. Vora, E. Faure, L. S. Thomas, E. T. Arnold, and M. Arditi. 2001. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J. Immunol. 1671609-1616. - PubMed
- Akhtar, M., J. L. Watson, A. Nazli, and D. M. McKay. 2003. Bacterial DNA evokes epithelial IL-8 production by a MAPK-dependent, NF-κB-independent pathway. FASEB J. 171319-1321. - PubMed
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2675-680. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources