HPV16 and BPV1 infection can be blocked by the dynamin inhibitor dynasore - PubMed (original) (raw)
HPV16 and BPV1 infection can be blocked by the dynamin inhibitor dynasore
Cynthia Y Abban et al. Am J Ther. 2008 Jul-Aug.
Abstract
The initial entry of papillomaviruses into their target cells has been shown to occur by clathrin-mediated endocytosis and caveolae-mediated endocytosis. These mechanisms entail the formation of nascent-coated vesicles at the plasma membrane. Such coated vesicles, clathrin or caveolin, form and pinch-off in a controlled mechanism that involves several proteins including dynamin. Dynamin is a GTPase that forms a dynamin ring at the stem connecting the nascent vesicle to the plasma membrane. In a still not fully characterized mechanism, dynamin's contraction and twisting results in the scission of the vesicle. In an effort to better characterize the role and molecular mechanisms of dynamin's function, researchers have identified dynasore, a dynamin GTPase inhibitor that prevents the scission of dynamin-dependent endocytic vesicles. Here, we have tested if infection by pseudovirus corresponding to the oncogenic human papillomavirus type 16 and bovine papillomavirus type 1 can be blocked by dynasore. We present data demonstrating that dynasore can block infection of human papillomavirus type 16 and bovine papillomavirus type 1 pseudovirions in a dose- and time-dependent manner with equal efficiency. Presently, there is no available therapy that can block infection by a wide range of papillomavirus regardless of species or genotypes. Targeting dynamin may lead to the rational design of drug able to prevent infection by papillomaviruses, and by other infectious agents dependent on this protein for initial internalization into target cells. Whether such an approach will prove successful needs further investigation.
Figures
FIGURE 1
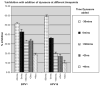
Dose-dependent inhibition of PV infection by dynasore inhibitor. HEK 293 cells were infected with BPV1 or HPV16 pseudovirions carrying the GFP cDNA, in the presence of 0, 20, 40, 80, and 100 μM dynasore. Infection was measured by FACS analysis of GFP-positive cells. Line with diamond is the BPV1 dose–response curve, and line with the rectangle is the HPV16 dose–response curve.
FIGURE 2
Dynasore inhibition of viral infection depends on the time of addition of the inhibitor. Dynasore (80 μM/mL) was added to 293 cells at different times during infection, and percent inhibition was measured by FACS analysis of GFP-positive cells. Bars: −30 minutes, dynasore was added 30 minutes before adding the virus; 0 minutes, dynasore was added just before incubating cells at 37°C; +30 minutes, dynasore was added 30 minutes after incubation at 37°C; +2 hours, dynasore was added 2 hours after infection; +4 hours, dynasore was added 4 hours after infection. Each bar is representative of independent experiments carried out in triplicates.
FIGURE 3
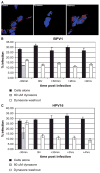
The inhibitory effect of dynasore is partly reversible. (A) Control panel: HEK 293 cells incubated with alexa-fluor 594–labeled transferrin; dynasore panel: cells were incubated with 80 μM/mL dynasore for 30 minutes and then labeled transferrin was added to media; washout panel: cells were incubated with 80 μM/mL dynasore for 30 minutes, media were replaced with dynasore-free fresh media, and labeled transferrin was added after 20 minutes. All steps were incubated at 37°C. Remaining surface transferrin was removed by acid wash before fixation and imaging of cells. Transferrin in red, and nuclei in blue (DAPI). (B andC)Black bars: percent infection of cells incubated with DMSO for 30 minutes and infected with BPV1 in B, HPV16 in C virus; gray bars: percent infection of cells incubated with dynasore for 30 minutes and infected with BPV1 virus; white bars: percent infection of cells incubated with dynasore for 30 minutes and infected with BPV1 virus. Dynasore was washed off (white bars) at −30 minutes, 0 hour, +30 minutes, +2 hours, and +4 hours, in relation addition of virus.
FIGURE 4
Electron microscopic analysis of the effect of dynasore inhibitor. (A) HEK 293 cells incubated for 30 minutes with 80 μM dynasore. (B–E) HEK 293 cells infected with BPV1 pseudovirus for 1 hour in the absence of dynasore. (F–I) HEK 293 cells infected with BPV1 pseudovirus for 1 hour in the presence of 80 μM dynasore. Scale bar represents 100 nm; ×80,000 magnification.
Similar articles
- Dynasore, a cell-permeable inhibitor of dynamin.
Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Macia E, et al. Dev Cell. 2006 Jun;10(6):839-50. doi: 10.1016/j.devcel.2006.04.002. Dev Cell. 2006. PMID: 16740485 - Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis.
Kirchhausen T, Macia E, Pelish HE. Kirchhausen T, et al. Methods Enzymol. 2008;438:77-93. doi: 10.1016/S0076-6879(07)38006-3. Methods Enzymol. 2008. PMID: 18413242 Free PMC article. - A highly-sensitive high throughput assay for dynamin's basal GTPase activity.
Mohanakrishnan A, Tran TVM, Kumar M, Chen H, Posner BA, Schmid SL. Mohanakrishnan A, et al. PLoS One. 2017 Sep 28;12(9):e0185639. doi: 10.1371/journal.pone.0185639. eCollection 2017. PLoS One. 2017. PMID: 28957392 Free PMC article. - Dynasore - not just a dynamin inhibitor.
Preta G, Cronin JG, Sheldon IM. Preta G, et al. Cell Commun Signal. 2015 Apr 10;13:24. doi: 10.1186/s12964-015-0102-1. Cell Commun Signal. 2015. PMID: 25889964 Free PMC article. Review. - Participation of dynamin in the biogenesis of cytoplasmic vesicles.
Henley JR, Cao H, McNiven MA. Henley JR, et al. FASEB J. 1999 Dec;13 Suppl 2:S243-7. doi: 10.1096/fasebj.13.9002.s243. FASEB J. 1999. PMID: 10619136 Review.
Cited by
- Human papillomavirus infection is inhibited by host autophagy in primary human keratinocytes.
Griffin LM, Cicchini L, Pyeon D. Griffin LM, et al. Virology. 2013 Mar 1;437(1):12-9. doi: 10.1016/j.virol.2012.12.004. Epub 2013 Jan 4. Virology. 2013. PMID: 23290079 Free PMC article. - Using Mesoporous Silica-Based Dual Biomimetic Nano-Erythrocytes for an Improved Antitumor Effect.
Xi Z, Jiang Y, Ma Z, Li Q, Xi X, Fan C, Zhu S, Zhang J, Xu L. Xi Z, et al. Pharmaceutics. 2023 Dec 15;15(12):2785. doi: 10.3390/pharmaceutics15122785. Pharmaceutics. 2023. PMID: 38140125 Free PMC article. - Infection of liver sinusoidal endothelial cells with Muromegalovirus muridbeta1 involves binding to neuropilin-1 and is dynamin-dependent.
Kyrrestad I, Larsen AK, Sánchez Romano J, Simón-Santamaría J, Li R, Sørensen KK. Kyrrestad I, et al. Front Cell Infect Microbiol. 2023 Nov 9;13:1249894. doi: 10.3389/fcimb.2023.1249894. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38029264 Free PMC article. - Entry of Newcastle Disease Virus into the host cell: role of acidic pH and endocytosis.
Sánchez-Felipe L, Villar E, Muñoz-Barroso I. Sánchez-Felipe L, et al. Biochim Biophys Acta. 2014 Jan;1838(1 Pt B):300-9. doi: 10.1016/j.bbamem.2013.08.008. Epub 2013 Aug 28. Biochim Biophys Acta. 2014. PMID: 23994097 Free PMC article. - Cholesterol-rich microdomains as docking platforms for respiratory syncytial virus in normal human bronchial epithelial cells.
San-Juan-Vergara H, Sampayo-Escobar V, Reyes N, Cha B, Pacheco-Lugo L, Wong T, Peeples ME, Collins PL, Castaño ME, Mohapatra SS. San-Juan-Vergara H, et al. J Virol. 2012 Feb;86(3):1832-43. doi: 10.1128/JVI.06274-11. Epub 2011 Nov 16. J Virol. 2012. PMID: 22090136 Free PMC article.
References
- Ehrlich M, Boll W, Van Oijen A, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. - PubMed
- Gaidarov I, Santini F, Warren RA, et al. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. - PubMed
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. - PubMed
- Pelkmans L, Helenius A. Insider information: what viruses tell us about endocytosis. Curr Opin Cell Biol. 2003;15:414–422. - PubMed
- van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources