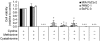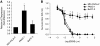The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance - PubMed (original) (raw)
The xc- cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance
M Lo et al. Br J Cancer. 2008.
Abstract
The x(c)(-) cystine transporter enhances biosynthesis of glutathione, a tripeptide thiol important in drug resistance and cellular defense against oxidative stress, by enabling cellular uptake of cystine, a rate-limiting precursor. Because it is known to regulate glutathione levels and growth of various cancer cell types, and is expressed in the pancreas, we postulate that it is involved in growth and drug resistance of pancreatic cancer. To examine this, we characterised expression of the x(c)(-) transporter in pancreatic cancer cell lines, MIA PaCa-2, PANC-1 and BxPC-3, as subjected to cystine-depletion and oxidative stress. The results indicate that these cell lines depend on x(c)(-)-mediated cystine uptake for growth, as well as survival in oxidative stress conditions, and can modulate x(c)(-) expression to accommodate growth needs. Immunohistochemical analysis showed that the transporter was differentially expressed in normal pancreatic tissues and overexpressed in pancreatic cancer tissues from two patients. Furthermore, gemcitabine resistance of cells was associated with elevated x(c)(-) expression and specific x(c)(-) inhibition by monosodium glutamate led to growth arrest. The results suggest that the x(c)(-) transporter by enhancing glutathione biosynthesis plays a major role in pancreatic cancer growth, therapy resistance and represents a potential therapeutic target for the disease.
Figures
Figure 1
Pancreatic cancer cells depend on extracellular cystine for growth. Neutral red uptake assay for cell proliferation in MIA PaCa-2, PANC-1, and BxPC-3 cells incubated in medium in the presence or absence of methionine (0.1 m
M
), cystine (0.1 m
M
), and/or cystathionine (0.15 m
M
), in the combinations indicated for 72 h. Data represent the mean±s.e.m. from three independent experiments. *P<0.01.
Figure 2
A negative correlation exists between extracellular cystine deprivation and expression of the xc− transporter. (A) Q-RT–PCR for expression of xCT or 4F2hc mRNA in MIA PaCa-2, PANC-1, and BxPC-3 cell lines incubated with various cystine concentrations (0.01, 0.1 or 1 m
M
) for 72 h. Data represent the mean±s.e.m. from three independent experiments. *_P_⩽0.05. (B) Western blot for expression of 4F2hc or tubulin in MIA PaCa-2, PANC-1, and BxPC-3 cell lines incubated with various cystine concentrations (0.01, 0.1 or 1 m
M
) for 72 h. Results are representative of two independent experiments.
Figure 3
Oxidative stress increases xc− transporter expression and GSH levels. (A) Intracellular GSH levels in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 m
M
DEM for 24 h. Data represent the mean±s.e.m. from at least two independent experiments. *P<0.05 using the Wilcoxon rank-sum test. (B) Q-RT–PCR for xCT and 4F2hc mRNA expression in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 m
M
DEM for 24 h. Data represent the mean±s.e.m. from at least two independent experiments. *P<0.05 (C) Western blot for expression of 4F2hc and tubulin in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 m
M
DEM for 24 h. Results are representative of two independent experiments. (D) Immunofluorescence for xCT (red) and DAPI (blue) in MIA PaCa-2, PANC-1, and BxPC-3 cell lines either untreated or treated with 1 m
M
DEM for 24 h. Results are representative of two independent experiments.
Figure 4
Expression of the xc− transporter in primary human pancreatic cancer specimens. Immunofluorescence for xCT (red) and DAPI (blue) in normal ductal cells, normal acinar cells, and pancreatic ductal adenocarcinoma cells from two primary human pancreatic cancer patient specimens.
Figure 5
A positive correlation exists between the expression level of xCT and resistance towards GEM. (A) Q-RT–PCR for xCT expression in MIA PaCa-2, PANC-1, and BxPC-3 cell lines. Data represent the mean±s.e.m. from three independent experiments. *P<0.05. (B) Neutral red uptake assay for cell proliferation in Mia PaCa-2, PANC-1, and BxPC-3 cultures treated with increasing concentrations of GEM for 72 h. Data represent the mean±s.e.m. from three independent experiments. *P<0.01.
Figure 6
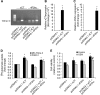
Overexpression of transfected xCT increases cystine uptake and increases GEM resistance. (A) RT–PCR for xCT and 4F2hc expression in MIA PaCa-2 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, or pcDNA3.1-4F2hc plasmid. (B, C) Q-RT–PCR for xCT (B) or 4F2hc (C) expression in MIA PaCa-2 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, or pcDNA3.1-4F2hc plasmid. *P<0.001. (D) [3H]-glutamate uptake assay in MIA PaCa-2 and PANC-1 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, and/or pcDNA3.1-4F2hc plasmid. *P<0.01 compared with respective cell line pcDNA3.1 control. (E) Neutral red uptake assay for survival of untreated or GEM-treated MIA PaCa-2 cells transfected with pcDNA3.1 empty vector, pcDNA3.1-xCT plasmid, and/or pcDNA3.1-4F2hc plasmid. *_P_⩽0.05 compared with pcDNA3.1 control; #_P_⩽0.05 compared with pcDNA3.1 GEM treatment. All data represent the mean±s.e.m. from three independent experiments.
Figure 7
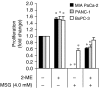
Targeting xc− transporter function with MSG. Neutral red uptake assay for cell proliferation in MIA PaCa-2, PANC-1, and BxPC-3 cultures treated with MSG (4.0 m
M
) or 2-ME (66 μ
M
) in various combinations for 72 h. *_P_⩽0.05. All data represent the mean±s.e.m. from three independent experiments.
Similar articles
- Pharmacogenomic approach reveals a role for the x(c)- cystine/glutamate antiporter in growth and celastrol resistance of glioma cell lines.
Pham AN, Blower PE, Alvarado O, Ravula R, Gout PW, Huang Y. Pham AN, et al. J Pharmacol Exp Ther. 2010 Mar;332(3):949-58. doi: 10.1124/jpet.109.162248. Epub 2009 Dec 9. J Pharmacol Exp Ther. 2010. PMID: 20007406 - ZIP4 Increases Expression of Transcription Factor ZEB1 to Promote Integrin α3β1 Signaling and Inhibit Expression of the Gemcitabine Transporter ENT1 in Pancreatic Cancer Cells.
Liu M, Zhang Y, Yang J, Cui X, Zhou Z, Zhan H, Ding K, Tian X, Yang Z, Fung KA, Edil BH, Postier RG, Bronze MS, Fernandez-Zapico ME, Stemmler MP, Brabletz T, Li YP, Houchen CW, Li M. Liu M, et al. Gastroenterology. 2020 Feb;158(3):679-692.e1. doi: 10.1053/j.gastro.2019.10.038. Epub 2019 Nov 9. Gastroenterology. 2020. PMID: 31711924 Free PMC article. - Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer.
Lo M, Ling V, Low C, Wang YZ, Gout PW. Lo M, et al. Curr Oncol. 2010 Jun;17(3):9-16. doi: 10.3747/co.v17i3.485. Curr Oncol. 2010. PMID: 20567622 Free PMC article. - Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target.
Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Albrecht P, et al. CNS Neurol Disord Drug Targets. 2010 Jul;9(3):373-82. doi: 10.2174/187152710791292567. CNS Neurol Disord Drug Targets. 2010. PMID: 20053169 Review. - Cystine-glutamate antiporter xCT as a therapeutic target for cancer.
Liu L, Liu R, Liu Y, Li G, Chen Q, Liu X, Ma S. Liu L, et al. Cell Biochem Funct. 2021 Mar;39(2):174-179. doi: 10.1002/cbf.3581. Epub 2020 Aug 4. Cell Biochem Funct. 2021. PMID: 32749001 Review.
Cited by
- Increased Expression of System xc- in Glioblastoma Confers an Altered Metabolic State and Temozolomide Resistance.
Polewski MD, Reveron-Thornton RF, Cherryholmes GA, Marinov GK, Cassady K, Aboody KS. Polewski MD, et al. Mol Cancer Res. 2016 Dec;14(12):1229-1242. doi: 10.1158/1541-7786.MCR-16-0028. Epub 2016 Sep 22. Mol Cancer Res. 2016. PMID: 27658422 Free PMC article. - Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy.
Koppula P, Zhuang L, Gan B. Koppula P, et al. Protein Cell. 2021 Aug;12(8):599-620. doi: 10.1007/s13238-020-00789-5. Epub 2020 Oct 1. Protein Cell. 2021. PMID: 33000412 Free PMC article. Review. - Immunoregulation through membrane proteins modified by reducing conditions induced by immune reactions.
Stegmann M, Metcalfe C, Barclay AN. Stegmann M, et al. Eur J Immunol. 2013 Jan;43(1):15-21. doi: 10.1002/eji.201242849. Eur J Immunol. 2013. PMID: 23233323 Free PMC article. Review. - Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma.
Hu K, Li K, Lv J, Feng J, Chen J, Wu H, Cheng F, Jiang W, Wang J, Pei H, Chiao PJ, Cai Z, Chen Y, Liu M, Pang X. Hu K, et al. J Clin Invest. 2020 Apr 1;130(4):1752-1766. doi: 10.1172/JCI124049. J Clin Invest. 2020. PMID: 31874110 Free PMC article. - Statin administration activates system xC- in skeletal muscle: a potential mechanism explaining statin-induced muscle pain.
Rebalka IA, Cao AW, May LL, Tarnopolsky MA, Hawke TJ. Rebalka IA, et al. Am J Physiol Cell Physiol. 2019 Nov 1;317(5):C894-C899. doi: 10.1152/ajpcell.00308.2019. Epub 2019 Sep 11. Am J Physiol Cell Physiol. 2019. PMID: 31509447 Free PMC article.
References
- Bannai S (1984a) Induction of cystine and glutamate transport activity in human fibroblasts by diethyl maleate and other electrophilic agents. J Biol Chem 259: 2435–2440 - PubMed
- Bannai S (1984b) Transport of cystine and cysteine in mammalian cells. Biochim Biophys Acta 779: 289–306 - PubMed
- Bannai S (1986) Exchange of cystine and glutamate across plasma membrane of human fibroblasts. J Biol Chem 261: 2256–2263 - PubMed
- Bassi MT, Gasol E, Manzoni M, Pineda M, Riboni M, Martin R, Zorzano A, Borsani G, Palacin M (2001) Identification and characterisation of human xCT that co-expresses, with 4F2 heavy chain, the amino acid transport activity system xc. Pflugers Arch 442: 286–296 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical