Paxillin comes of age - PubMed (original) (raw)
Review
Paxillin comes of age
Nicholas O Deakin et al. J Cell Sci. 2008.
Abstract
Paxillin is a multi-domain scaffold protein that localizes to the intracellular surface of sites of cell adhesion to the extracellular matrix. Through the interactions of its multiple protein-binding modules, many of which are regulated by phosphorylation, paxillin serves as a platform for the recruitment of numerous regulatory and structural proteins that together control the dynamic changes in cell adhesion, cytoskeletal reorganization and gene expression that are necessary for cell migration and survival. In particular, paxillin plays a central role in coordinating the spatial and temporal action of the Rho family of small GTPases, which regulate the actin cytoskeleton, by recruiting an array of GTPase activator, suppressor and effector proteins to cell adhesions. When paxillin was first described 18 years ago, the amazing complexity of cell-adhesion organization, dynamics and signaling was yet to be realized. Herein we highlight our current understanding of how the multiple protein interactions of paxillin contribute to the coordination of cell-adhesion function.
Figures
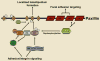
Fig. 1. Focal adhesions provide both a structural and signaling link between the extracellular matrix and the actin cytoskeleton
Cell adhesion to the extracellular matrix, via transmembrane integrin αβ heterodimers, leads to integrin activation and the recruitment of numerous intracellular proteins to the plasma membrane. Focal adhesions now comprise over 125 proteins (only selected examples are depicted), including both structural and regulatory molecules that mediate a physical link to the actin cytoskeleton and also play a major role in regulating actin dynamics necessary for productive cell migration. Molecules such as paxillin serve as scaffold proteins to facilitate the functional integration of these different categories of focal adhesion proteins.
Fig. 2. The LD4 motif of paxillin regulates cell protrusion and retraction
CHO.K1 cells stably transfected with (A) wild-type paxillin or (B.) a mutant of paxillin that lacks the LD4 motif (ΔLD4) were spread on 10μg/ml fibronectin for 2 hours in the presence of serum. Cells were fixed and stained for F-actin (red) and paxillin (green). Deletion of the LD4 domain of paxillin results in extensive peripheral protrusive activity (arrows) and defective tail retraction (arrowhead). It is also of note that the protrusions observed in cells that express paxillin ΔLD4 contain numerous dot-like paxillin-rich focal complexes at their periphery, which are less prevalent in cells that express wild-type paxillin. Scale bar = 20 μm.
Fig. 3. The coordination of Rac signaling by the paxillin LD4 motif
Paxillin is one of the earliest proteins that is recruited to nascent focal adhesions and is necessary for the turnover of focal adhesions during cell migration. The engagement of integrins with the extracellular matrix results in the localized activation of Rac and the Src and FAK tyrosine kinases. Together, Rac, Src and FAK promote the recruitment of the GIT-PIX-PAK-NCK complex to focal adhesions by means of an interaction between the paxillin binding subdomain 2 (PBS2) of GIT2 and the paxillin LD4 motif. Rac mediates this process by binding to and activating its effector PAK, which stimulates a multi-step conformational remodeling of the GIT-PIX-PAK-NCK complex and the PAK-dependent phosphorylation of the paxillin LD4 motif at S273. Src-FAK-dependent phosphorylation of GIT is also necessary for its binding to paxillin. The Rac GEF activity of PIX might function in a feed-forward loop to further promote localized Rac signaling to PAK at the cell’s leading edge. The recruitment of the GIT-PIX-PAK-NCK complex to paxillin also serves as a termination signal for Rac activity. This is also regulated at multiple levels, including the PTP-PEST-dependent tyrosine dephosphorylation of GIT, paxillin and FAK, as well as the GIT-dependent inhibition of Arf6 signaling to Rac (not shown). The serine/threonine phosphatase PP2A might also be recruited to paxillin to dephosphorylate S273.
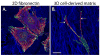
Fig. 4. Paxillin is enriched in adhesions of fibroblasts migrating on either 2D and 3D fibronectin matrices
Mouse embryonic fibroblasts (MEFs) were allowed to migrate for 16 hours in the presence of serum on (A) a 2D fibronectin-coated surface or (B) a 3D cell-derived fibronectin-rich matrix (CDM) that is more representative of the in vivo environment. Cells were fixed and stained for F-actin (red), paxillin (green) and fibronectin (blue). Although paxillin-rich adhesions are observed in both conditions, MEFs migrating on the 2D fibronectin are well-spread and exhibit robust actin stress fibers, whereas cells migrating within the 3D CDM display long, thin protrusions (arrows) and limited actin organization. Scale bar = 20μm.
Similar articles
- Paxillin phosphorylation at serine 273 and its effects on Rac, Rho and adhesion dynamics.
Tang K, Boudreau CG, Brown CM, Khadra A. Tang K, et al. PLoS Comput Biol. 2018 Jul 5;14(7):e1006303. doi: 10.1371/journal.pcbi.1006303. eCollection 2018 Jul. PLoS Comput Biol. 2018. PMID: 29975690 Free PMC article. - Emerging role of paxillin-PKL in regulation of cell adhesion, polarity and migration.
Yu JA, Deakin NO, Turner CE. Yu JA, et al. Cell Adh Migr. 2010 Jul-Sep;4(3):342-7. doi: 10.4161/cam.4.3.11406. Epub 2010 Jul 3. Cell Adh Migr. 2010. PMID: 20372092 Free PMC article. - Paxillin S273 Phosphorylation Regulates Adhesion Dynamics and Cell Migration through a Common Protein Complex with PAK1 and βPIX.
Rajah A, Boudreau CG, Ilie A, Wee TL, Tang K, Borisov AZ, Orlowski J, Brown CM. Rajah A, et al. Sci Rep. 2019 Aug 7;9(1):11430. doi: 10.1038/s41598-019-47722-3. Sci Rep. 2019. PMID: 31391572 Free PMC article. - Paxillin: a crossroad in pathological cell migration.
López-Colomé AM, Lee-Rivera I, Benavides-Hidalgo R, López E. López-Colomé AM, et al. J Hematol Oncol. 2017 Feb 18;10(1):50. doi: 10.1186/s13045-017-0418-y. J Hematol Oncol. 2017. PMID: 28214467 Free PMC article. Review. - The explorations of dynamic interactions of paxillin at the focal adhesions.
Aziz AUR, Deng S, Jin Y, Li N, Zhang Z, Yu X, Liu B. Aziz AUR, et al. Biochim Biophys Acta Proteins Proteom. 2022 Oct 1;1870(10):140825. doi: 10.1016/j.bbapap.2022.140825. Epub 2022 Aug 1. Biochim Biophys Acta Proteins Proteom. 2022. PMID: 35926716 Review.
Cited by
- Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth.
Thievessen I, Thompson PM, Berlemont S, Plevock KM, Plotnikov SV, Zemljic-Harpf A, Ross RS, Davidson MW, Danuser G, Campbell SL, Waterman CM. Thievessen I, et al. J Cell Biol. 2013 Jul 8;202(1):163-77. doi: 10.1083/jcb.201303129. J Cell Biol. 2013. PMID: 23836933 Free PMC article. - Regulation of paxillin-p130-PI3K-AKT signaling axis by Src and PTPRT impacts colon tumorigenesis.
Zhao Y, Scott A, Zhang P, Hao Y, Feng X, Somasundaram S, Khalil AM, Willis J, Wang Z. Zhao Y, et al. Oncotarget. 2017 Jul 25;8(30):48782-48793. doi: 10.18632/oncotarget.10654. Oncotarget. 2017. PMID: 27447856 Free PMC article. - Roles of paxillin family members in adhesion and ECM degradation coupling at invadosomes.
Petropoulos C, Oddou C, Emadali A, Hiriart-Bryant E, Boyault C, Faurobert E, Vande Pol S, Kim-Kaneyama JR, Kraut A, Coute Y, Block M, Albiges-Rizo C, Destaing O. Petropoulos C, et al. J Cell Biol. 2016 Jun 6;213(5):585-99. doi: 10.1083/jcb.201510036. J Cell Biol. 2016. PMID: 27269065 Free PMC article. - Phosphatase and Tensin Homolog (PTEN) Represses Colon Cancer Progression through Inhibiting Paxillin Transcription via PI3K/AKT/NF-κB Pathway.
Zhang LL, Mu GG, Ding QS, Li YX, Shi YB, Dai JF, Yu HG. Zhang LL, et al. J Biol Chem. 2015 Jun 12;290(24):15018-29. doi: 10.1074/jbc.M115.641407. Epub 2015 Apr 14. J Biol Chem. 2015. PMID: 25873394 Free PMC article. - Cytohesin-2/ARNO, through its interaction with focal adhesion adaptor protein paxillin, regulates preadipocyte migration via the downstream activation of Arf6.
Torii T, Miyamoto Y, Sanbe A, Nishimura K, Yamauchi J, Tanoue A. Torii T, et al. J Biol Chem. 2010 Jul 30;285(31):24270-81. doi: 10.1074/jbc.M110.125658. Epub 2010 Jun 4. J Biol Chem. 2010. PMID: 20525696 Free PMC article.
References
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67:359–67. - PubMed
- Ando R, Mizuno H, Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science. 2004;306:1370–3. - PubMed
- Arold ST, Hoellerer MK, Noble ME. The structural basis of localization and signaling by the focal adhesion targeting domain. Structure (Camb) 2002;10:319–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM047607/GM/NIGMS NIH HHS/United States
- R01 HL070244/HL/NHLBI NIH HHS/United States
- GM47607/GM/NIGMS NIH HHS/United States
- HL070244/HL/NHLBI NIH HHS/United States
- R01 GM047607-17/GM/NIGMS NIH HHS/United States
- R01 HL070244-06/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources