Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis - PubMed (original) (raw)
. 2008 Aug 20;27(16):2181-93.
doi: 10.1038/emboj.2008.149. Epub 2008 Jul 24.
Pablo J Fernandez-Marcos, Anita Galvez, Ramars Amanchy, Juan F Linares, Angeles Duran, Peterson Pathrose, Michael Leitges, Marta Cañamero, Manuel Collado, Clara Salas, Manuel Serrano, Jorge Moscat, Maria T Diaz-Meco
Affiliations
- PMID: 18650932
- PMCID: PMC2519103
- DOI: 10.1038/emboj.2008.149
Par-4 inhibits Akt and suppresses Ras-induced lung tumorigenesis
Jayashree Joshi et al. EMBO J. 2008.
Abstract
The atypical PKC-interacting protein, Par-4, inhibits cell survival and tumorigenesis in vitro, and its genetic inactivation in mice leads to reduced lifespan, enhanced benign tumour development and low-frequency carcinogenesis. Here, we demonstrate that Par-4 is highly expressed in normal lung but reduced in human lung cancer samples. We show, in a mouse model of lung tumours, that the lack of Par-4 dramatically enhances Ras-induced lung carcinoma formation in vivo, acting as a negative regulator of Akt activation. We also demonstrate in cell culture, in vivo, and in biochemical experiments that Akt regulation by Par-4 is mediated by PKCzeta, establishing a new paradigm for Akt regulation and, likely, for Ras-induced lung carcinogenesis, wherein Par-4 is a novel tumour suppressor.
Figures
Figure 1
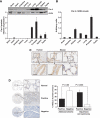
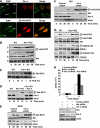
Par-4 is highly expressed in the lung and is downregulated in human lung tumours. (A) Par-4 protein expression in normal mouse tissues. (B) Par-4 mRNA levels in normal mouse tissues. (C) Par-4 distribution in human and mouse tissues, determined by IHC. (D) Commercial tissue microarrays of NSCLC human samples (_n_=133) were stained with anti-Par-4 antibody. Par-4 expression was analysed in adenocarcinoma and squamous cell carcinoma tumours compared with normal lung tissue. A representative example of positive and negative tumour samples and normal control for Par-4 staining is shown at two magnifications ( × 2.5 and × 20). Scale bar=50 μm.
Figure 2
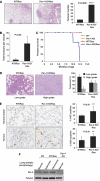
Par-4 cooperates with Ras-induced tumorigenesis in the lung. (A) H&E staining of lungs from WT and Par-4 KO mice crossed with K-ras+/V12, RERT2T/T mice and analysed 5 months after activation of the K-rasV12 allele by injection of 4-hydroxytamoxifen (_n_=5 per genotype). Overall tumour burden was determined by quantification of the tumour area as a percentage of total area of H&E-stained tissue (right panel). (B) Number of total tumours per mouse in WT and Par-4 KO mice. (C) Survival of mice of different genotypes represented as percentage of total; the cause of death was asphyxiation. (D) Loss of Par-4 leads to increased Ras-induced high-grade lung adenocarcinomas. (E) Representative sections and quantification of proliferation index measured as percentage of positive nuclear staining for Ki67 in lung sections from normal alveolar or tumour tissue from WT and Par-4 KO Ras-expressing mice. (F) Par-4 expression levels in the lung from different mouse genotypes. **, P<0.01; ****, P<0.0001.
Figure 3
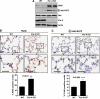
Increased levels of XIAP and activation of RelA and Akt in lungs of Par-4 KO mice. (A) The levels of XIAP, phospho-Akt-S473, Akt, Par-4 and actin were determined in lung extracts from WT and Par-4 KO mice. These are representative experiments where there were at least two others with similar results. (B, C) Sections of alveolar lung tissue from WT and Par-4 KO mice were stained by IHC with anti-RelA (B) or anti-phospho-Akt-S473 (C) antibody and scored for the number of cells with nuclear staining. Results are the mean±s.d. of 10 different fields per mouse, with a total of five mice for each condition. Scale bar=50 μm. **, P<0.01; ****, P<0.003.
Figure 4
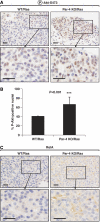
Increased activation of Akt but not RelA in Ras-expressing Par-4 KO lung tumours. Sections of lung tumours from WT and Par-4 KO mice expressing Ras were stained by IHC with anti-phospho-Akt-S473 (A) or anti-RelA (C) antibody. (B) Quantitation of cells showing positive nuclear staining for phospho-Akt-S473. Results are the mean±s.d. of 10 different fields per mouse, with a total of five mice for each condition. Scale bar=50 μm. ***, P<0.001.
Figure 5
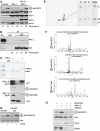
Par-4 deficiency induces increased nuclear phospho-Akt in vivo. (A) Confocal immunofluorescence on WT and Par-4 KO EFs seeded on the same coverslip, double-stained for Par-4 and XIAP (upper panels), or XIAP and phospho-Akt-S473 (lower panels). WT and Par-4 KO EFs stimulated with serum (FCS) for different durations or a dose–response for 15 min (C) in total extracts (C, D) or in nuclear and cytosol extracts (B). Reconstitution of Par-4 KO EFs with Par-4 restored phospho-Akt-S473 levels to basal levels (D). (E, F) A549 or 293 cells treated with a control siRNA or with Par-4-specific siRNA were stimulated with serum for different times and the levels of phospho-Akt-S473 were determined. Knockdown of Par-4 was confirmed by immunoblot. (G) WT and Par-4 KO EFs stimulated with serum were analysed by immunoblot for phospho-Akt-S473 and phospho-Akt-T308 levels as well as the Akt substrates, Gsk3β-S9 and Foxo3-T32. (H) Knockdown of Akt blocks the increased cell proliferation induced by knockdown of Par-4 in A549 cells. Cell number was determined by trypan blue exclusion. Knockdown of Par-4 and Akt was analysed by immunoblot. These are representative experiments where there were at least two others with similar results.
Figure 6
PKCζ directly interacts and phosphorylates Akt. (A) Overexpression of Par-4 in 293 cells inhibited serum-induced phospho-Akt-S473 phosphorylation and was reversed by PKCζ co-expression. (B) Serum-stimulated 293 cells extracts were immunoprecipitated with anti-Akt or IgG control antibody and the immunoprecipitates were analysed by immunoblot with anti-PKCζ or anti-Akt, as a loading control. (C) In vitro phosphorylation of recombinant Akt by recombinant PKCζ with γP32-ATP. Part of the assay reaction was also immunoblotted with anti-phospho-Akt-Ser473, anti-phospho-Akt-T308 and anti-Akt antibodies. (D) Immunoprecipitates of WT or KD Akt were phosphorylated in vitro by PKCζ and phospho-Akt-Ser473 levels were determined. (E) Phosphopeptide map of in vitro PKCζ phosphorylated-Akt (left panel). Phospho-amino-acid analysis of peptides A, B, C and the total reaction. (F) MS/MS spectra of the identified phosphopeptides corresponding to sites S124, S473 and T308 phosphorylated in Akt by PKCζ. (G) A549 cells treated with a control siRNA, Par-4–siRNA, Rictor-siRNA or both siRNAs were stimulated with serum and the levels of phospho-Akt-S473 were determined. Knockdown of Par-4 and Rictor was confirmed by immunoblot. These are representative experiments where there were at least two others with similar results.
Figure 7
Activation of Akt by Par-4 deficiency is dependent on PKCζ in vivo. (A) WT and PKCζ KO EFs stimulated with serum were analysed by immunoblot for phospho-Akt-S473 and phospho-Akt-T308 levels. (B) Lung extracts from WT and PKCζ KO were immunoblotted with XIAP, phospho-Akt-S473 and phospho-Akt-T308 antibodies. (C–E) Phosphorylation of Akt in Par-4 KO is reverted in DKO (Par-4/PKCζ). Phosphorylation of Akt and its substrate FOXO3 were determined in lung extracts of the different KO mice (C). Lung sections were stained for phospho-Akt-S473. Quantitation of cells showing positive nuclear staining for phospho-Akt-S473 (D). Results are the mean±s.d. of 10 different fields per mouse with a total of five mice for each condition. (E) Lung sections were stained for phospho-Akt-S124. Negative control was performed on Par-4 KO sample with no primary antibody. These are representative experiments where there were at least two others with similar results. Scale=50 μm.
Similar articles
- Akt regulation and lung cancer: a novel role and mechanism of action for the tumor suppressor Par-4.
Diaz-Meco MT, Moscat J. Diaz-Meco MT, et al. Cell Cycle. 2008 Sep 15;7(18):2817-20. doi: 10.4161/cc.7.18.6735. Epub 2008 Sep 5. Cell Cycle. 2008. PMID: 18769154 - TERT and Akt Are Involved in the Par-4-Dependent Apoptosis of Islet β Cells in Type 2 Diabetes.
Liu C, QiNan W, XiaoTian L, MengLiu Y, XiaGuang G, WeiLing L, ZiWen L, Ling Z, GangYi Y, Bing C. Liu C, et al. J Diabetes Res. 2018 Aug 14;2018:7653904. doi: 10.1155/2018/7653904. eCollection 2018. J Diabetes Res. 2018. PMID: 30186877 Free PMC article. - Protein kinase C delta is required for survival of cells expressing activated p21RAS.
Xia S, Forman LW, Faller DV. Xia S, et al. J Biol Chem. 2007 May 4;282(18):13199-210. doi: 10.1074/jbc.M610225200. Epub 2007 Mar 8. J Biol Chem. 2007. PMID: 17350960 Free PMC article. - Mechanisms of apoptosis by the tumor suppressor Par-4.
Hebbar N, Wang C, Rangnekar VM. Hebbar N, et al. J Cell Physiol. 2012 Dec;227(12):3715-21. doi: 10.1002/jcp.24098. J Cell Physiol. 2012. PMID: 22552839 Free PMC article. Review. - Apoptosis and tumor resistance conferred by Par-4.
Zhao Y, Rangnekar VM. Zhao Y, et al. Cancer Biol Ther. 2008 Dec;7(12):1867-74. doi: 10.4161/cbt.7.12.6945. Epub 2008 Dec 8. Cancer Biol Ther. 2008. PMID: 18836307 Free PMC article. Review.
Cited by
- PAR-4 overcomes chemo-resistance in breast cancer cells by antagonizing cIAP1.
Guo H, Treude F, Krämer OH, Lüscher B, Hartkamp J. Guo H, et al. Sci Rep. 2019 Jun 19;9(1):8755. doi: 10.1038/s41598-019-45209-9. Sci Rep. 2019. PMID: 31217499 Free PMC article. - The Par-4/PTEN connection in tumor suppression.
Diaz-Meco MT, Abu-Baker S. Diaz-Meco MT, et al. Cell Cycle. 2009 Aug 15;8(16):2518-22. doi: 10.4161/cc.8.16.9384. Epub 2009 Aug 29. Cell Cycle. 2009. PMID: 19625770 Free PMC article. Review. - Fbxo45 joins the 'Par-4'ty in controlling apoptosis of cancer cells.
Wang Z, Wei W. Wang Z, et al. Cell Death Differ. 2014 Oct;21(10):1508-10. doi: 10.1038/cdd.2014.104. Cell Death Differ. 2014. PMID: 25196972 Free PMC article. No abstract available. - Secretory prostate apoptosis response (Par)-4 sensitizes multicellular spheroids (MCS) of glioblastoma multiforme cells to tamoxifen-induced cell death.
Jagtap JC, Parveen D, Shah RD, Desai A, Bhosale D, Chugh A, Ranade D, Karnik S, Khedkar B, Mathur A, Natesh K, Chandrika G, Shastry P. Jagtap JC, et al. FEBS Open Bio. 2014 Nov 21;5:8-19. doi: 10.1016/j.fob.2014.11.005. eCollection 2015. FEBS Open Bio. 2014. PMID: 25685660 Free PMC article. - AKT is indispensable for coordinating Par-4/JNK cross talk in p21 downmodulation during ER stress.
Rasool RU, Nayak D, Chakraborty S, Faheem MM, Rah B, Mahajan P, Gopinath V, Katoch A, Iqra Z, Yousuf SK, Mukherjee D, Kumar LD, Nargotra A, Goswami A. Rasool RU, et al. Oncogenesis. 2017 May 22;6(5):e341. doi: 10.1038/oncsis.2017.41. Oncogenesis. 2017. PMID: 28530706 Free PMC article.
References
- Asara JM, Christofk HR, Freimark LM, Cantley LC (2008) A label-free quantification method by MS/MS TIC compared to SILAC and spectral counting in a proteomics screen. Proteomics 8: 994–999 - PubMed
- Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, McCormick F, Feng J, Tsichlis P (1998) Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene 17: 313–325 - PubMed
- Bhaskar PT, Hay N (2007) The Two TORCs and Akt. Dev Cell 12: 487–502 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases