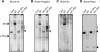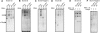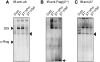Dissecting beta-ring assembly pathway of the mammalian 20S proteasome - PubMed (original) (raw)
Dissecting beta-ring assembly pathway of the mammalian 20S proteasome
Yuko Hirano et al. EMBO J. 2008.
Abstract
The 20S proteasome is the catalytic core of the 26S proteasome. It comprises four stacked rings of seven subunits each, alpha(1-7)beta(1-7)beta(1-7)alpha(1-7). Recent studies indicated that proteasome-specific chaperones and beta-subunit appendages assist in the formation of alpha-rings and dimerization of half-proteasomes, but the process involved in the assembly of beta-rings is poorly understood. Here, we clarify the mechanism of beta-ring formation on alpha-rings by characterizing assembly intermediates accumulated in cells depleted of each beta-subunit. Starting from beta2, incorporation of beta-subunits occurs in an orderly manner dependent on the propeptides of beta2 and beta5, and the C-terminal tail of beta2. Unexpectedly, hUmp1, a chaperone functioning at the final assembly step, is incorporated as early as beta2 and is required for the structural integrity of early assembly intermediates. We propose a model in which beta-ring formation is assisted by both intramolecular and extrinsic chaperones, whose roles are partially different between yeast and mammals.
Figures
Figure 1
Accumulation of distinct assembly intermediates in each β-subunit knockdown cells. The cell extracts (40 μg) used in Supplementary Figure S1 were separated by native PAGE. Assembly intermediates were detected by immunoblotting using the indicated antibodies (A, C–I) The bands corresponding to α-ring and the 20S proteasome as well as the locations of molecular size markers are depicted by arrowheads. (B) The peptide-hydrolysing activity was assayed by active staining of the gel using Suc-LLVY-MCA in the presence of SDS. Note that the 26S proteasome did not move inside the native PAGE gel.
Figure 2
Release of PAC3 is coupled with β3 incorporation. The same panels in Figure 1 were probed with anti-PAC1 (A) and -PAC3 (B) antibodies. The arrow indicates PAC3 species dissociated from proteasome precursors (B).
Figure 3
Role of hUmp1 in the structural integrity of early assembly intermediates. (A) The same panel in Figure 1 was probed with anti-hUmp1 antibody. (B–E) Extracts of HEK293T cells transfected with the indicated combinations of siRNAs were separated by native PAGE. Intermediate complexes were detected by immunoblotting using the indicated antibodies. The left lane representing β7 RNAi serves as a positive control for immunoblotting (C, D). Asterisks indicate nonspecific bands (A). (F) Flag–hUmp1 and each 20S subunit were co-translated and radio-labelled in reticulocyte lysates, immunoprecipitated with M2 agarose, and analysed by SDS–PAGE and autoradiography. ‘ALL' represents co-translation of all β-subunits together with hUmp1.
Figure 4
Both the propeptide and C-terminal tail of β2 are indispensable for β3 incorporation. Stable cell lines expressing the indicated mutant β2-subunits were treated with the siRNA targeting endogenous β2. Intermediate complexes were detected by immunoblotting using the indicated antibodies following native PAGE (A–D). Intermediates observed in β2*ΔTail cells can be divided into two species; faster migrating ones (arrowheads) and slower migrating ones (vertical bars). The free complex of PAC3 is depicted by an arrow (D).
Figure 5
β5 propeptide is required for β6 incorporation. Stable cell lines expressing the indicated mutant β5-subunits were treated with the siRNA(s) for endogenous β5 or β6 (A–D), or for the indicated combinations (E, F). Cell extracts were resolved by native PAGE, followed by immunoblot analysis for the indicated antibodies.
Figure 6
C-terminal tail of β7 is essential for the incorporation of β7 and dimerization of half-mers. Stable cell lines expressing the indicated mutant β7-subunits were treated with siRNA targeting endogenous β7. Cell extracts were resolved by native PAGE, followed by immunoblot analysis using the indicated antibodies. The free form of β7*Δtail is depicted by an arrow (B). The ‘half-mer' assembly intermediates are depicted by arrowheads (A, C).
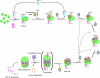
Figure 7
A model for β-ring formation in mammalian 20S proteasome assembly. The roles of PAC1–PAC2 and PAC3 in the formation of α-rings were described previously (Hirano et al, 2005, 2006). PAC4 was recently identified as a heterodimeric partner of PAC3 (Le Tallec et al, 2007). Sequential incorporation of β-subunits starts from the association of β2 and hUmp1 on the α-ring. hUmp1 is required for the association of β2 in the early assembly intermediates. PAC3–PAC4, whose release is coupled with association of β3, holds the structural integrity of the intermediates until β3 is incorporated on the α-ring. Subsequent orderly incorporation of other β-subunits is also assisted by intramolecular chaperones such as the propeptides of β2 and β5 and the C-terminal tail of β2. Although β1 can be incorporated at various steps (dotted lines), such incorporation most likely follows that of β6. Dimerization of half-mers is assisted by the C-terminal tail of β7. This is followed by removal of β-subunit propeptides (β1, β2, β5, β6, and β7) and hUmp1 degradation. Essential propeptides, non-essential propeptides, and essential C-terminal tails of β-subunits for mammalian 20S proteasome biogenesis are depicted in red, blue, and yellow, respectively. See text for more details.
Similar articles
- Cooperation of multiple chaperones required for the assembly of mammalian 20S proteasomes.
Hirano Y, Hayashi H, Iemura S, Hendil KB, Niwa S, Kishimoto T, Kasahara M, Natsume T, Tanaka K, Murata S. Hirano Y, et al. Mol Cell. 2006 Dec 28;24(6):977-84. doi: 10.1016/j.molcel.2006.11.015. Mol Cell. 2006. PMID: 17189198 - A heterodimeric complex that promotes the assembly of mammalian 20S proteasomes.
Hirano Y, Hendil KB, Yashiroda H, Iemura S, Nagane R, Hioki Y, Natsume T, Tanaka K, Murata S. Hirano Y, et al. Nature. 2005 Oct 27;437(7063):1381-5. doi: 10.1038/nature04106. Nature. 2005. PMID: 16251969 - Chaperone-driven proteasome assembly.
Rosenzweig R, Glickman MH. Rosenzweig R, et al. Biochem Soc Trans. 2008 Oct;36(Pt 5):807-12. doi: 10.1042/BST0360807. Biochem Soc Trans. 2008. PMID: 18793141 Review. - Chaperone-assisted assembly of the proteasome core particle.
Matias AC, Ramos PC, Dohmen RJ. Matias AC, et al. Biochem Soc Trans. 2010 Feb;38(Pt 1):29-33. doi: 10.1042/BST0380029. Biochem Soc Trans. 2010. PMID: 20074030 - PACemakers of proteasome core particle assembly.
Ramos PC, Dohmen RJ. Ramos PC, et al. Structure. 2008 Sep 10;16(9):1296-304. doi: 10.1016/j.str.2008.07.001. Structure. 2008. PMID: 18786393 Review.
Cited by
- siRNA silencing of proteasome maturation protein (POMP) activates the unfolded protein response and constitutes a model for KLICK genodermatosis.
Dahlqvist J, Törmä H, Badhai J, Dahl N. Dahlqvist J, et al. PLoS One. 2012;7(1):e29471. doi: 10.1371/journal.pone.0029471. Epub 2012 Jan 3. PLoS One. 2012. PMID: 22235297 Free PMC article. - Precise assembly and regulation of 26S proteasome and correlation between proteasome dysfunction and neurodegenerative diseases.
Im E, Chung KC. Im E, et al. BMB Rep. 2016 Sep;49(9):459-73. doi: 10.5483/bmbrep.2016.49.9.094. BMB Rep. 2016. PMID: 27312603 Free PMC article. Review. - In-depth Analysis of the Lid Subunits Assembly Mechanism in Mammals.
Bai M, Zhao X, Sahara K, Ohte Y, Hirano Y, Kaneko T, Yashiroda H, Murata S. Bai M, et al. Biomolecules. 2019 May 31;9(6):213. doi: 10.3390/biom9060213. Biomolecules. 2019. PMID: 31159305 Free PMC article. - Assembly mechanisms of specialized core particles of the proteasome.
Bai M, Zhao X, Sahara K, Ohte Y, Hirano Y, Kaneko T, Yashiroda H, Murata S. Bai M, et al. Biomolecules. 2014 Jul 16;4(3):662-77. doi: 10.3390/biom4030662. Biomolecules. 2014. PMID: 25033340 Free PMC article. - Identification of survival genes in human glioblastoma cells by small interfering RNA screening.
Thaker NG, Zhang F, McDonald PR, Shun TY, Lewen MD, Pollack IF, Lazo JS. Thaker NG, et al. Mol Pharmacol. 2009 Dec;76(6):1246-55. doi: 10.1124/mol.109.058024. Epub 2009 Sep 25. Mol Pharmacol. 2009. PMID: 19783622 Free PMC article.
References
- Adams J (2004) The proteasome: a suitable antineoplastic target. Nat Rev Cancer 4: 349–360 - PubMed
- Baumeister W, Walz J, Zuhl F, Seemuller E (1998) The proteasome: paradigm of a self-compartmentalizing protease. Cell 92: 367–380 - PubMed
- Chen P, Hochstrasser M (1996) Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell 86: 961–972 - PubMed
- Chondrogianni N, Tzavelas C, Pemberton AJ, Nezis IP, Rivett AJ, Gonos ES (2005) Overexpression of proteasome beta5 assembled subunit increases the amount of proteasome and confers ameliorated response to oxidative stress and higher survival rates. J Biol Chem 280: 11840–11850 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources