Control of cytoplasmic maturation events by cytomegalovirus tegument protein pp150 - PubMed (original) (raw)
Control of cytoplasmic maturation events by cytomegalovirus tegument protein pp150
Ritesh Tandon et al. J Virol. 2008 Oct.
Abstract
Cytomegalovirus replication depends upon a betaherpesvirus-conserved 150-kDa tegument phosphoprotein (pp150; encoded by UL32) that supports the final steps in virion maturation at cytoplasmic assembly compartments. Amino acid substitutions were introduced into conserved region 1 (CR1) and CR2 of pp150, affecting a region that may interact with nucleocapsids. Two independent CR2 point mutants (N201A and G207A) failed to support viral replication in evaluations by a transient complementation assay or after reconstruction into recombinant viruses. An assembly compartment-like cytoplasmic inclusion developed in UL32 mutant virus-infected cells that was similar to that of wild-type virus-infected cells. The cellular localization of the trans-Golgi marker Golgin-97 suggested differences in the organization of the assembly compartment compared to that of wild-type virus-infected cells. Replication-defective CR2 point mutants exhibited the same phenotype as that of a virus carrying a complete deletion of the UL32 open reading frame in these assays. Electron micrographs of fibroblasts at 3 or 5 days postinfection with a deletion mutant (DeltaUL32) grown on UL32-complementing cells showed a similar number and morphology of capsids in the nucleus, but the cytoplasmic region associated with virion assembly appeared highly vesiculated and contained few recognizable nucleocapsids or complete virus particles. These data demonstrate that the principle role of pp150 is to retain nucleocapsid organization through secondary envelopment at the assembly compartment.
Figures
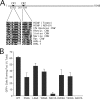
FIG. 1.
(A) Line diagram of pp150 (1,048 aa) illustrating amino-terminal CR1 (L52 to Y62) and CR2 (N201 to L209). The alignment of amino acid sequences of CR1 and CR2 from different betaherpesviruses is shown in the expanded region. The Towne and AD169 strains of HCMV are aligned with chimpanzee, rhesus, simian, mouse, and rat CMV species and also with human herpesviruses-6 and -7. (B) Secondary spread assay evaluation of plasmids expressing WT or mutant forms of UL32 to complement ΔUL32-BAC. Individual point mutations in CR1 (F53A, L56A, and W59A) and CR2 (N201A, K202A, Y205A, and G207A) were tested by cotransfection with ΔUL32-BAC DNA in HFs.
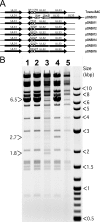
FIG. 2.
(A) Schematic representation of UL32 mutations introduced into the Towne-BAC genome using lambda-red-mediated recombination. The portion of UL32 containing CR1 and CR2 was replaced with a Kanr-SacB cassette (pON5010) that was used to derive pON5011-pON5016 bacmids as shown. (B) Electrophoretic separation of HindIII-digested BAC DNAs on agarose gel (0.8%) as a diagnostic for the correct insertion of the Kanr-SacB cassette and the integrity of BAC genome. Digests of G207A-BAC (lane 1), N201A-BAC (lane 2), ΔUL32-BAC (lane 3), ΔUL32-Kanr-SacB-BAC (lane 4), and Towne-BAC (lane 5) are shown. The presence of the pSIM6 plasmid (6.5 kb) confers lambda red functions to these BACs. This plasmid was lost during the growth of bacterial culture in the absence of ampicillin (lanes 3 and 5). The 2.7-kb band results from the insertion of the Kanr-SacB cassette, and the 1.8-kb band results from insertion of the Kanr cassette in the Towne-BAC genome.
FIG. 3.
Replication properties of BAC-reconstituted replication-competent viruses. (A to J) Fluorescent images of eGFP-positive cells at day 10 posttransfection of HFs with Towne-BAC, ΔUL32-BAC, ΔCR1ΔCR2-BAC, rescued (R) CR1CR2-BAC, RUL32-BAC, F53A-BAC, W59A-BAC, N201A-BAC, K202A-BAC, and G207A-BAC. (K) Single-step growth curves (MOI of 5.0) of Towne-BAC and replication-competent UL32 mutant BAC-derived recombinant viruses. Virus titers were determined by plaque assays of supernatants collected daily. The standard deviation from the mean of each titer is within the symbols.
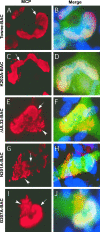
FIG. 4.
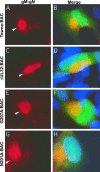
Immunofluorescence localization of MCP in HFs transfected with Towne-BAC (A, B), K202A-BAC (C, D), ΔUL32-BAC (E, F), N201A-BAC (G, H), or G207A-BAC (I, J). HFs were fixed 9 days posttransfection and stained with mouse monoclonal anti-MCP primary antibody and anti-mouse Texas red secondary antibody (A, C, E, G, and I) before immunofluorescence imaging at ×1,000 magnification. Hoechst 33258 staining was used to identify nuclei, in merged images that include contributions from Texas red and virus-encoded eGFP (B, D, F, H, and J). Arrows point to nuclear MCP in all panels, and arrowheads point to the cytoplasmic MCP detected in ΔUL32-BAC-, N201A-BAC-, or G207A-BAC-transfected cells in which pp150 is nonfunctional.
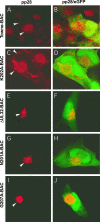
FIG. 5.
Immunofluorescent localization of pp28 to the AC in HFs transfected with Towne-BAC (A, B), K202A-BAC (C, D), ΔUL32-BAC (E, F), N201A-BAC (G, H), or G207A-BAC (I, J). HFs were fixed 9 days posttransfection and stained with mouse monoclonal anti-pp28 primary antibody and anti-mouse Texas red secondary antibody (A, C, E, G, and I) before immunofluorescence imaging at ×1,000 magnification. Arrowheads point to pp28 within the AC and cytoplasms of transfected cells. Merged images include contributions from Texas red and eGFP (B, D, F, H, and J).
FIG. 6.
Immunofluorescent localization of gM:gN in HFs transfected with Towne-BAC (A, B), ΔUL32-BAC (C, D), G207A-BAC (E, F), or N201A-BAC (G, H). HFs were fixed 9 days posttransfection and stained with mouse monoclonal 14-16A primary antibody against gM:gN complex and anti-mouse Texas red secondary antibody (A, C, E, and G) before immunofluorescence imaging at ×1,000 magnification. Arrowheads point to gM:gN within the AC detected in Towne-BAC and K202A-BAC infections in which pp150 is functional and also in ΔUL32-BAC-, N201A-BAC-, or G207A-BAC-transfected cells in which pp150 is nonfunctional. Hoechst 33258 staining was used to identify nuclei, which are depicted as merged images that include contributions from Texas red and eGFP (B, D, F, and H).
FIG. 7.
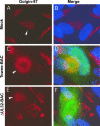
Immunofluorescence localization of the _trans_-Golgi network in HFs. Either mock-transfected (Mock) (A, B), Towne-BAC-transfected (C, D), or ΔUL32-BAC-transfected (E, F) HFs were fixed 9 days posttransfection and stained with mouse monoclonal anti-Golgin-97 primary antibody and anti-mouse Texas red secondary antibody before imaging at ×1,000 magnification. Arrowheads mark the _trans_-Golgi network within individual cells.
FIG. 8.
Transmission electron micrographs of HFs illustrating differences between Towne-BAC and ΔUL32 virus infections at day 3 (A through F) and day 5 (G through L) postinfection. Nuc, nucleus; Cyt, cytoplasm. Black arrows, C capsids; black arrowheads, dense bodies; white arrows, empty capsids; white arrowheads, B capsids.
FIG. 8.
Transmission electron micrographs of HFs illustrating differences between Towne-BAC and ΔUL32 virus infections at day 3 (A through F) and day 5 (G through L) postinfection. Nuc, nucleus; Cyt, cytoplasm. Black arrows, C capsids; black arrowheads, dense bodies; white arrows, empty capsids; white arrowheads, B capsids.
Similar articles
- Betaherpesvirus-conserved cytomegalovirus tegument protein ppUL32 (pp150) controls cytoplasmic events during virion maturation.
AuCoin DP, Smith GB, Meiering CD, Mocarski ES. AuCoin DP, et al. J Virol. 2006 Aug;80(16):8199-210. doi: 10.1128/JVI.00457-06. J Virol. 2006. PMID: 16873276 Free PMC article. - Cytomegalovirus pUL96 is critical for the stability of pp150-associated nucleocapsids.
Tandon R, Mocarski ES. Tandon R, et al. J Virol. 2011 Jul;85(14):7129-41. doi: 10.1128/JVI.02549-10. Epub 2011 May 18. J Virol. 2011. PMID: 21593167 Free PMC article. - C Proteins: Controllers of Orderly Paramyxovirus Replication and of the Innate Immune Response.
Siering O, Cattaneo R, Pfaller CK. Siering O, et al. Viruses. 2022 Jan 12;14(1):137. doi: 10.3390/v14010137. Viruses. 2022. PMID: 35062341 Free PMC article. Review. - Virus-encoded homologs of cellular interleukin-10 and their control of host immune function.
Slobedman B, Barry PA, Spencer JV, Avdic S, Abendroth A. Slobedman B, et al. J Virol. 2009 Oct;83(19):9618-29. doi: 10.1128/JVI.01098-09. Epub 2009 Jul 29. J Virol. 2009. PMID: 19640997 Free PMC article. Review. No abstract available.
Cited by
- Dynamin Is Required for Efficient Cytomegalovirus Maturation and Envelopment.
Hasan MH, Davis LE, Bollavarapu RK, Mitra D, Parmar R, Tandon R. Hasan MH, et al. J Virol. 2018 Nov 27;92(24):e01418-18. doi: 10.1128/JVI.01418-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282704 Free PMC article. - Role of tegument proteins in herpesvirus assembly and egress.
Guo H, Shen S, Wang L, Deng H. Guo H, et al. Protein Cell. 2010 Nov;1(11):987-98. doi: 10.1007/s13238-010-0120-0. Epub 2010 Dec 10. Protein Cell. 2010. PMID: 21153516 Free PMC article. Review. - Role of human cytomegalovirus tegument proteins in virion assembly.
Smith RM, Kosuri S, Kerry JA. Smith RM, et al. Viruses. 2014 Feb 6;6(2):582-605. doi: 10.3390/v6020582. Viruses. 2014. PMID: 24509811 Free PMC article. Review. - The tegument protein pp65 of human cytomegalovirus acts as an optional scaffold protein that optimizes protein uploading into viral particles.
Reyda S, Tenzer S, Navarro P, Gebauer W, Saur M, Krauter S, Büscher N, Plachter B. Reyda S, et al. J Virol. 2014 Sep 1;88(17):9633-46. doi: 10.1128/JVI.01415-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920816 Free PMC article. - Virus inhibition of RIP3-dependent necrosis.
Upton JW, Kaiser WJ, Mocarski ES. Upton JW, et al. Cell Host Microbe. 2010 Apr 22;7(4):302-313. doi: 10.1016/j.chom.2010.03.006. Cell Host Microbe. 2010. PMID: 20413098 Free PMC article.
References
- Azzeh, M., A. Honigman, A. Taraboulos, A. Rouvinski, and D. G. Wolf. 2006. Structural changes in human cytomegalovirus cytoplasmic assembly sites in the absence of UL97 kinase activity. Virology 35469-79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources