Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity - PubMed (original) (raw)
Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity
Wei Yu et al. EMBO Rep. 2008 Sep.
Abstract
In multicellular epithelial tissues, the orientation of polarity of each cell must be coordinated. Previously, we reported that for Madin-Darby canine kidney cells in three-dimensional collagen gel culture, blockade of beta1-integrin by the AIIB2 antibody or expression of dominant-negative Rac1N17 led to an inversion of polarity, such that the apical surfaces of the cells were misorientated towards the extracellular matrix. Here, we show that this process results from the activation of RhoA. Knockdown of RhoA by short hairpin RNA reverses the inverted orientation of polarity, resulting in normal cysts. Inhibition of RhoA downstream effectors, Rho kinase (ROCK I) and myosin II, has similar effects. We conclude that the RhoA-ROCK I-myosin II pathway controls the inversion of orientation of epithelial polarity caused by AIIB2 or Rac1N17. These results might be relevant to the hyperactivation of RhoA and disruption of normal polarity frequently observed in human epithelial cancers.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
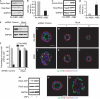
Loss of RhoA function rescues the phenotype caused by AIIB2 treatment. (A,B) Treatment with AIIB2 decreases Rac1 activity, measured by GST-Pak3-CRIB pull-down assay. (C,D) RhoA activity is assessed by a GST-RBD-Rhotekin pull-down assay. (E) Reduction of RhoA with shRNA in two clones shown by immunoblot. (F) Quantification of cysts with normal polarity in cells transfected with control shRNA or RhoA shRNA in the absence or presence of AIIB2. A total of 100 cysts per condition were counted from three experiments; *P<0.001. (G–L) Representative confocal images. The two selected clones were plated in COLI for 5 days. Normal polarity was determined by gp135 (green) at the luminal surface and by β-catenin (red) at the BL surface; nuclei are shown in blue. Scale bars 20 μm. (M–O) Activation of RhoA causes inverted cysts. (M) Activation of RhoA by CNFy treatment is assessed by GST-Rhotekin pull-down assay. CNFy results in cysts with inverted polarity, which was indicated by gp135 at the peripheral surface (green) and β-catenin (red) at the BL surface (O, compare normal cyst in N); nuclei are shown in blue. a.u., arbitrary units; BL, basolateral; COLI, collagen I; CNFy, Yersinia pseudotuberculosis cytotoxic necrotizing factor; CRIB, Cdc42/Rac interactive binding; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GST, glutathione _S_-transferase; Pak3, p21 activated kinase 3; RBD, Ras binding domain; shRNA, short hairpin RNA.
Figure 2
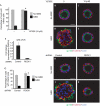
Depletion of ROCK I rescues AIIB2 polarity. (A) Quantification of cysts with normal polarity in cells treated with Y27632; *P<0.001. (B–E) Representative confocal images of cysts stained with gp135 (green) and β-catenin (red); nuclei are shown in blue. (F–K) Depletion of ROCK I rescues AIIB2-induced phenotype. (F) Reduction of ROCK I expression level by RNAi is confirmed by QRT–PCR. (G) Quantification of cysts with normal polarity in cells transfected with shRNA to ROCK I; _n_=3; *P<0.001. (H–J) Confocal images of representative cysts. Cells infected with lentivirus for control or ROCK I were plated in COLI for 5 days and stained with gp135 (green) and β-catenin (red); nuclei are shown in blue. Scale bars, 20 μm. COLI, collagen I; QRT–PCR, quantitative reverse transcription–PCR; RNAi, RNA interference; ROCK, Rho kinase; shRNA, short hairpin RNA.
Figure 3
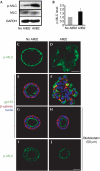
Inhibition of myosin activity rescues the phenotype induced by AIIB2 treatment. (A,B) MLC activity was analysed by Western blot of cysts for p-MLC against rabbit p-MLC at Ser 19. GAPDH acts as a loading control. (C,D) Confocal micrographs of p-MLC. (C) In normal cysts, p-MLC is localized mainly at the basal plasma membrane, some p-MLC are in the region of TJ. (D) In AIIB2-treated cysts, p-MLC is mislocalized. (E–J) Inhibition of myosin activity by blebbistatin rescues AIIB2-induced phenotype, as indicated by the apical marker gp135 (green in H) located at the luminal surface, and β-catenin (red in H) located peripherally and at the cell–cell contact (compare F). Note that blebbistatin treatment results in normal distribution of p-MLC in the presence of AIIB2 (J). Scale bars, 20 μm. MLC, myosin light chain; p-MLC, phosphorylation of MLC; TJ, tight junction.
Figure 4
Inhibition of ROCK or myosin II reverts Rac1N17 cysts. MDCK cells were stably transfected with tetracycline-repressible Myc-Rac1N17, which is expressed by removal of doxycycline in (B,D,F,H). (A,B) Immunostaining of Myc (green) confirms expression of exogenous Rac1N17 in (B) compared with negative control in (A). Inverted polarity is indicated by gp135 at the periphery and β-catenin at the cell–cell contact when Rac1N17 is expressed (D), but not in control cysts (C). Inhibition of ROCK by Y27632 (F) or myosin activity by blebbistatin (H) reverts the inverted orientation of polarity. (C–H) gp135 is green, β-catenin is red and nuclei are blue. (I) Quantification of polarity in Rac1N17 cysts by treatment with Y27632 or blebbistatin; *P<0.001. Scale bar, 10 μm. MDCK, Madin–Darby canine kidney cells; ROCK, Rho kinase.
Similar articles
- p114RhoGEF governs cell motility and lumen formation during tubulogenesis through a ROCK-myosin-II pathway.
Kim M, M Shewan A, Ewald AJ, Werb Z, Mostov KE. Kim M, et al. J Cell Sci. 2015 Dec 1;128(23):4317-27. doi: 10.1242/jcs.172361. Epub 2015 Oct 19. J Cell Sci. 2015. PMID: 26483385 Free PMC article. - The RhoA-Rok-myosin II pathway is involved in extracellular matrix-mediated regulation of prolactin signaling in mammary epithelial cells.
Du JY, Chen MC, Hsu TC, Wang JH, Brackenbury L, Lin TH, Wu YY, Yang Z, Streuli CH, Lee YJ. Du JY, et al. J Cell Physiol. 2012 Apr;227(4):1553-60. doi: 10.1002/jcp.22886. J Cell Physiol. 2012. PMID: 21678418 Free PMC article. - Cell confinement controls centrosome positioning and lumen initiation during epithelial morphogenesis.
Rodríguez-Fraticelli AE, Auzan M, Alonso MA, Bornens M, Martín-Belmonte F. Rodríguez-Fraticelli AE, et al. J Cell Biol. 2012 Sep 17;198(6):1011-23. doi: 10.1083/jcb.201203075. Epub 2012 Sep 10. J Cell Biol. 2012. PMID: 22965908 Free PMC article. - Inhibitors of the ROCK serine/threonine kinases: key effectors of the RhoA small GTPase.
Vigil D, Der CJ. Vigil D, et al. Enzymes. 2013;33 Pt A:193-212. doi: 10.1016/B978-0-12-416749-0.00009-9. Epub 2013 Aug 8. Enzymes. 2013. PMID: 25033806 Review. - Abnormality of apico-basal polarity in adenocarcinoma.
Onuma K, Inoue M. Onuma K, et al. Cancer Sci. 2022 Nov;113(11):3657-3663. doi: 10.1111/cas.15549. Epub 2022 Sep 14. Cancer Sci. 2022. PMID: 36047965 Free PMC article. Review.
Cited by
- Mechanistic Insights into Colorectal Cancer Phenomics from Fundamental and Organotypic Model Studies.
Campbell FC, Loughrey MB, McClements J, Deevi RK, Javadi A, Rainey L. Campbell FC, et al. Am J Pathol. 2018 Sep;188(9):1936-1948. doi: 10.1016/j.ajpath.2018.05.021. Epub 2018 Jul 18. Am J Pathol. 2018. PMID: 30028958 Free PMC article. Review. - Dynamics of salivary gland morphogenesis.
Harunaga J, Hsu JC, Yamada KM. Harunaga J, et al. J Dent Res. 2011 Sep;90(9):1070-7. doi: 10.1177/0022034511405330. Epub 2011 Apr 12. J Dent Res. 2011. PMID: 21487116 Free PMC article. Review. - Arg kinase regulates epithelial cell polarity by targeting β1-integrin and small GTPase pathways.
Li R, Pendergast AM. Li R, et al. Curr Biol. 2011 Sep 27;21(18):1534-42. doi: 10.1016/j.cub.2011.08.023. Epub 2011 Sep 8. Curr Biol. 2011. PMID: 21906945 Free PMC article. - Laminins in Epithelial Cell Polarization: Old Questions in Search of New Answers.
Matlin KS, Myllymäki SM, Manninen A. Matlin KS, et al. Cold Spring Harb Perspect Biol. 2017 Oct 3;9(10):a027920. doi: 10.1101/cshperspect.a027920. Cold Spring Harb Perspect Biol. 2017. PMID: 28159878 Free PMC article. Review. - Dynamin Binding Protein (Tuba) Deficiency Inhibits Ciliogenesis and Nephrogenesis in Vitro and in Vivo.
Baek JI, Kwon SH, Zuo X, Choi SY, Kim SH, Lipschutz JH. Baek JI, et al. J Biol Chem. 2016 Apr 15;291(16):8632-43. doi: 10.1074/jbc.M115.688663. Epub 2016 Feb 19. J Biol Chem. 2016. PMID: 26895965 Free PMC article.
References
- Cowan CR, Hyman AA (2007) Acto-myosin reorganization and PAR polarity in C. elegans. Development 134: 1035–1043 - PubMed
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K (2006) Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 DK068358/DK/NIDDK NIH HHS/United States
- R01 GM076363/GM/NIGMS NIH HHS/United States
- R01 GM076363-04/GM/NIGMS NIH HHS/United States
- K08DK68358/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials