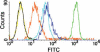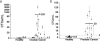Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands - PubMed (original) (raw)
Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands
Wei He et al. Int J Cancer. 2008.
Abstract
Quantitation of circulating tumor cells (CTCs) can provide information on the stage of a malignancy, onset of disease progression and response to therapy. In an effort to more accurately quantitate CTCs, we have synthesized fluorescent conjugates of 2 high-affinity tumor-specific ligands (folate-AlexaFluor 488 and DUPA-FITC) that bind tumor cells >20-fold more efficiently than fluorescent antibodies. Here we determine whether these tumor-specific dyes can be exploited for quantitation of CTCs in peripheral blood samples from cancer patients. A CTC-enriched fraction was isolated from the peripheral blood of ovarian and prostate cancer patients by an optimized density gradient centrifugation protocol and labeled with the aforementioned fluorescent ligands. CTCs were then quantitated by flow cytometry. CTCs were detected in 18 of 20 ovarian cancer patients (mean 222 CTCs/ml; median 15 CTCs/ml; maximum 3,118 CTCs/ml), whereas CTC numbers in 16 gender-matched normal volunteers were negligible (mean 0.4 CTCs/ml; median 0.3 CTCs/ml; maximum 1.5 CTCs/ml; p < 0.001, chi(2)). CTCs were also detected in 10 of 13 prostate cancer patients (mean 26 CTCs/ml, median 14 CTCs/ml, maximum 94 CTCs/ml) but not in 18 gender-matched healthy donors (mean 0.8 CTCs/ml, median 1, maximum 3 CTC/ml; p < 0.0026, chi(2)). Tumor-specific fluorescent antibodies were much less efficient in quantitating CTCs because of their lower CTC labeling efficiency. Use of tumor-specific fluorescent ligands to label CTCs in peripheral blood can provide a simple, accurate and sensitive method for determining the number of cancer cells circulating in the bloodstream.
Figures
Figure 1
Comparison of KB cell labeling intensity with folate-FITC versus various anti-FR antibodies. KB cells were labeled with (i) folate-FITC (green), (ii) affinity-purified polyclonal anti-FR antibody directly labeled with FITC (PU17-FITC, orange), (iii) monoclonal anti-FR antibody (mAb343, cyan) followed by FITC-labeled goat anti-mouse IgG or (iv) affinity-purified polyclonal anti-FR antibody (PU17, navy) followed by FITC-labeled rat anti-rabbit IgG prior to analysis by flow cytometry. Unlabeled KB cells (black) and KB cells incubated with 10 μM folic acid plus 100 nM folate-FITC (yellow, totally competed) served as negative controls.
Figure 2
CTC quantitation in blood samples from ovarian and prostate cancer patients. Dots in (a) and (b) represent mean values from multiple independent measurements of CTCs in blood samples from individual healthy subjects, or ovarian or prostate cancer patients. Bars in (a) and (b) indicate the average number of CTCs in the collection of blood samples from healthy donors, or ovarian or prostate cancer patients. Threshold values of 1.5 CTCs/ml for ovarian cancer samples and 3 CTC/ml for prostate cancer samples are indicated by dashed lines in (a) and (b).
Similar articles
- In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry.
He W, Wang H, Hartmann LC, Cheng JX, Low PS. He W, et al. Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11760-5. doi: 10.1073/pnas.0703875104. Epub 2007 Jun 29. Proc Natl Acad Sci U S A. 2007. PMID: 17601776 Free PMC article. - Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients.
Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, Matulonis UA. Liu JF, et al. Gynecol Oncol. 2013 Nov;131(2):352-6. doi: 10.1016/j.ygyno.2013.08.006. Epub 2013 Aug 13. Gynecol Oncol. 2013. PMID: 23954902 Clinical Trial. - Gene expression profiling of single circulating tumor cells in ovarian cancer - Establishment of a multi-marker gene panel.
Blassl C, Kuhlmann JD, Webers A, Wimberger P, Fehm T, Neubauer H. Blassl C, et al. Mol Oncol. 2016 Aug;10(7):1030-42. doi: 10.1016/j.molonc.2016.04.002. Epub 2016 Apr 20. Mol Oncol. 2016. PMID: 27157930 Free PMC article. - [Circulating tumor cells (CTCs)--clinical significance in patients with ovarian cancer].
Magnowski P, Bochyński H, Nowak-Markwitz E, Zabel M, Spaczyński M. Magnowski P, et al. Ginekol Pol. 2012 Apr;83(4):291-4. Ginekol Pol. 2012. PMID: 22712262 Review. Polish. - Cell labeling approaches for fluorescence-based in vivo flow cytometry.
Pitsillides CM, Runnels JM, Spencer JA, Zhi L, Wu MX, Lin CP. Pitsillides CM, et al. Cytometry A. 2011 Oct;79(10):758-65. doi: 10.1002/cyto.a.21125. Epub 2011 Sep 8. Cytometry A. 2011. PMID: 21905206 Free PMC article. Review.
Cited by
- Folate-Receptor Positive Circulating Tumor Cell Is a Potential Diagnostic Marker of Prostate Cancer.
Lian S, Yang L, Feng Q, Wang P, Wang Y, Li Z. Lian S, et al. Front Oncol. 2021 Oct 8;11:708214. doi: 10.3389/fonc.2021.708214. eCollection 2021. Front Oncol. 2021. PMID: 34692484 Free PMC article. - Liquid biopsies to distinguish malignant from benign pulmonary nodules.
Tao R, Cao W, Zhu F, Nie J, Wang H, Wang L, Liu P, Chen H, Hong B, Zhao D. Tao R, et al. Thorac Cancer. 2021 Jun;12(11):1647-1655. doi: 10.1111/1759-7714.13982. Epub 2021 May 7. Thorac Cancer. 2021. PMID: 33960710 Free PMC article. Review. - Principles in the design of ligand-targeted cancer therapeutics and imaging agents.
Srinivasarao M, Galliford CV, Low PS. Srinivasarao M, et al. Nat Rev Drug Discov. 2015 Mar;14(3):203-19. doi: 10.1038/nrd4519. Epub 2015 Feb 20. Nat Rev Drug Discov. 2015. PMID: 25698644 Review. - Preoperative Folate Receptor-Positive Circulating Tumor Cells Are Associated With Occult Peritoneal Metastasis and Early Recurrence in Gastric Cancer Patients: A Prospective Cohort Study.
Zeng CDD, Jin CC, Gao C, Xiao AT, Tong YX, Zhang S. Zeng CDD, et al. Front Oncol. 2022 Mar 29;12:769203. doi: 10.3389/fonc.2022.769203. eCollection 2022. Front Oncol. 2022. PMID: 35425708 Free PMC article. - Treatment for non-small-cell lung cancer and circulating tumor cells.
Mason J, Blyth B, MacManus MP, Martin OA. Mason J, et al. Lung Cancer Manag. 2017 Dec;6(4):129-139. doi: 10.2217/lmt-2017-0019. Epub 2018 Jun 22. Lung Cancer Manag. 2017. PMID: 30643579 Free PMC article. Review.
References
- Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975;35:512–6. - PubMed
- Donelli MG, Rosso R, Garattini S. Quantitative studies on cancer dissemination. Cancer Res. 1969;29:414–18. - PubMed
- Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 1974;34:997–1004. - PubMed
- Koike A. Mechanism of blood-borne metastases. I. Some factors affecting lodgment and growth of tumor cells in the lungs. Cancer. 1964;17:450–60. - PubMed
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical