Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4) - PubMed (original) (raw)
doi: 10.1111/j.1460-9568.2008.06321.x.
Yingning Zhang, Kimberley Brown, Benjamen D Coats, Mitesh Shridhar, Paige W Sholar, Sonica J Patel, Nicole Y Crysdale, Jacqueline A Harrison, Steven F Maier, Kenner C Rice, Linda R Watkins
Affiliations
- PMID: 18662331
- PMCID: PMC2588470
- DOI: 10.1111/j.1460-9568.2008.06321.x
Non-stereoselective reversal of neuropathic pain by naloxone and naltrexone: involvement of toll-like receptor 4 (TLR4)
Mark R Hutchinson et al. Eur J Neurosci. 2008 Jul.
Abstract
Although activated spinal cord glia contribute importantly to neuropathic pain, how nerve injury activates glia remains controversial. It has recently been proposed, on the basis of genetic approaches, that toll-like receptor 4 (TLR4) may be a key receptor for initiating microglial activation following L5 spinal nerve injury. The present studies extend this idea pharmacologically by showing that TLR4 is key for maintaining neuropathic pain following sciatic nerve chronic constriction injury (CCI). Established neuropathic pain was reversed by intrathecally delivered TLR4 receptor antagonists derived from lipopolysaccharide. Additionally, (+)-naltrexone, (+)-naloxone, and (-)-naloxone, which we show here to be TLR4 antagonists in vitro on both stably transfected HEK293-TLR4 and microglial cell lines, suppressed neuropathic pain with complete reversal upon chronic infusion. Immunohistochemical analyses of spinal cords following chronic infusion revealed suppression of CCI-induced microglial activation by (+)-naloxone and (-)-naloxone, paralleling reversal of neuropathic pain. Together, these CCI data support the conclusion that neuron-to-glia signaling through TLR4 is important not only for initiating neuropathic pain, as suggested previously, but also for maintaining established neuropathic pain. Furthermore, these studies suggest that the novel TLR4 antagonists (+)-naloxone and (-)-naloxone can each fully reverse established neuropathic pain upon multi-day administration. This finding with (+)-naloxone is of potential clinical relevance. This is because (+)-naloxone is an antagonist that is inactive at the (-)-opioid selective receptors on neurons that produce analgesia. Thus, these data suggest that (+)-opioid antagonists such as (+)-naloxone may be useful clinically to suppress glial activation, yet (-)-opioid agonists suppress pain.
Figures
Fig. 1
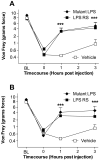
Reversal of chronic constriction injury (CCI)-induced neuropathic pain by acute intrathecal delivery of the toll-like receptor (TLR)4 antagonists, mutant lipopolysaccharide (LPS), and LPS-RS. After baseline (BL) testing, rats received CCI of one sciatic nerve at the mid-thigh level. After pre-drug testing (0 h) 14 days later to confirm the development of bilateral CCI-induced mechanical allodynia, rats were intrathecally given either 20 μg of mutant LPS (filled diamonds), 20 μg of LPS-RS (filled squares), or an equal volume of vehicle (open squares). Behavioral responses recorded 1 and 3 h later revealed reliable attenuation of both ipsilateral (A) and contralateral (B) mechanical allodynia by this TLR4 antagonist. ***P < 0.001 as compared to vehicle (saline) controls.
Fig. 2
Non-classic blockade of lipopolysaccharide (LPS)-induced toll-like receptor (TLR) signaling and microglial activation. HEK-TLR4 cells were incubated with LPS (log doses from 0 to 100 ng/mL) along with either vehicle (LPS control), 10 ng/mL LPS-RS (A), 10 μ
m
(+)-naltrexone (B), or 10 μ
m
(+)-naloxone (C). All drugs inhibited TLR4 signaling, as reflected by secreted alkaline phosphatase (SEAP) levels, with competitive antagonism observed using LPS-RS and non-competitive antagonism by (+)-naltrexone and (+)-naloxone on the basis of the shapes of the resultant curves. Comparable antagonisms were documented for (-))-naltrexone and (-))-naloxone, but are not included here for clarity. In a separate study, the HAPI microglial cell line was used to examine the effects of (+)-naloxone on microglial activation by LPS. Whereas 100 ng/mL LPS produced elevations in mRNA for CD11b (microglial activation marker) (E), interleukin (IL)-1β (D), and IL-6 (F), 1 μ
m
(+)-naloxone abolished each of these LPS-induced effects. GAPDH, glyceraldehyde-3-phosphate-dehydrogenase.
Fig. 3
Reversal of chronic constriction injury (CCI)-induced neuropathic pain by acute delivery of neuronally inactive (+)-naloxone and (+)-naltrexone, novel toll-like receptor (TLR)4 antagonists. After baseline (BL) testing, rats received CCI of one sciatic nerve at the mid-thigh level. After pre-drug testing (0 h) 14 days later to confirm the development of bilateral CCI-induced mechanical allodynia, rats were intrathecally (IT) given 60 μg of either (+)-naloxone (filled squares, A and B), (+)-naltrexone (filled circles, C and D), 100 mg/kg subcutaneous (+)-naloxone (filled squares, E and F) or vehicle (open symbols, A-F). Behavioral responses recorded after drug administration revealed reliable attenuation of both ipsilateral (A, C and E) and contralateral (B, D and F) mechanical allodynia by these novel TLR4 antagonists. *P < 0.05, **P < 0.01, ***P < 0.001 as compared to vehicle (saline) controls.
Fig. 4
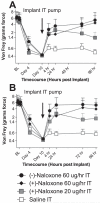
Persistent reversal of chronic constriction injury (CCI)-induced neuro-pathic pain by sustained intrathecal (IT) delivery of neuronally inactive (+)-naloxone and (+)-naltrexone. After baseline (BL) testing, rats received CCI of one sciatic nerve at the mid-thigh level. After behavioral assessments on days 4 and 10 to confirm the development of bilateral CCI-induced mechanical allodynia, rats were implanted with osmotic minipumps to produce sustained IT administration of either (-))-naloxone (filled circles, 60 μg/h), (+)-naloxone (filled diamonds, 60 μg/h), (+)-naloxone (filled squares, 20 μg/h), or vehicle (open squares). Behavioral responses recorded over time after drug administration revealed reliable attenuation of both ipsilateral (A) and contralateral (B) mechanical allodynia by these novel toll-like receptor 4 antagonists with complete reversal of allodynia by 60 μg/h (-))-naloxone and 60 μg/h (+)-naloxone by 96 h. Treatment effects compared to vehicle: P < 0.0001.
Fig. 5
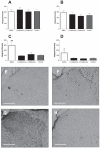
Sustained intrathecal delivery of neuronally inactive (+)-opioid antagonists suppresses the expression of a microglial activation marker. The L5 spinal cords of chronic constriction injury (CCI) rats receiving sustained intrathecal administration of 60 μg/h(-))-naloxone, 60 μg/h (+)-naloxone or vehicle for 4 days in Experiment 3 (behavior shown in Fig. 3) were analysed for expression of astrocyte [glial fibrillary acidic protein (GFAP); A and B] and microglia (CD11b/c; C-H) activation markers. As quantified by densitometry, neither (+)-naloxone nor (-))-naloxone affected GFAP expression in ipsilateral (A) or contralateral (B) dorsal horns, as compared to vehicle controls. In contrast, both isomers of naloxone produced reliable suppression of CD11b/c bilaterally, as compared to neuropathic vehicle-treated animals (C and D). Representative sections (10×) of ipsilateral dorsal horns of neuropathic (CCI) rats treated intrathecally with 60 μg/h (+)-naloxone (E), 60 μg/h(-))-naloxone (F) and vehicle (G) are shown. A comparable section from a naïve control is illustrated in H. *P < 0.05, **P < 0.01 as compared to naive controls. Scale bar: 500 μ
m
.
Similar articles
- (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats.
Lewis SS, Loram LC, Hutchinson MR, Li CM, Zhang Y, Maier SF, Huang Y, Rice KC, Watkins LR. Lewis SS, et al. J Pain. 2012 May;13(5):498-506. doi: 10.1016/j.jpain.2012.02.005. Epub 2012 Apr 20. J Pain. 2012. PMID: 22520687 Free PMC article. - Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4.
Wang X, Zhang Y, Peng Y, Hutchinson MR, Rice KC, Yin H, Watkins LR. Wang X, et al. Br J Pharmacol. 2016 Mar;173(5):856-69. doi: 10.1111/bph.13394. Epub 2016 Feb 4. Br J Pharmacol. 2016. PMID: 26603732 Free PMC article. - Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta.
Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR. Lewis SS, et al. Neuroscience. 2010 Jan 20;165(2):569-83. doi: 10.1016/j.neuroscience.2009.10.011. Neuroscience. 2010. PMID: 19833175 Free PMC article. - Non-analgesic effects of opioids: opioid-induced respiratory depression.
Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A. Boom M, et al. Curr Pharm Des. 2012;18(37):5994-6004. doi: 10.2174/138161212803582469. Curr Pharm Des. 2012. PMID: 22747535 Review. - [Molecular aspects of pharmacological activity of naltrexone and naloxone].
Golovko AI, Golovko SI, Leont'eva LV, Romanenko OI, Konoplin DA. Golovko AI, et al. Eksp Klin Farmakol. 2003 Jan-Feb;66(1):71-8. Eksp Klin Farmakol. 2003. PMID: 12683088 Review. Russian.
Cited by
- The influence of sex on neuroimmune communication, pain, and physiology.
Alexander SN, Green AR, Debner EK, Ramos Freitas LE, Abdelhadi HMK, Szabo-Pardi TA, Burton MD. Alexander SN, et al. Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review. - Naltrexone modulates contextual processing in depression.
Chen J, Mizuno A, Lyew T, Karim HT, Karp JF, Dombrovski AY, Peciña M. Chen J, et al. Neuropsychopharmacology. 2020 Nov;45(12):2070-2078. doi: 10.1038/s41386-020-00809-2. Epub 2020 Aug 25. Neuropsychopharmacology. 2020. PMID: 32843703 Free PMC article. Clinical Trial. - Pronociceptive and Antinociceptive Effects of Buprenorphine in the Spinal Cord Dorsal Horn Cover a Dose Range of Four Orders of Magnitude.
Gerhold KJ, Drdla-Schutting R, Honsek SD, Forsthuber L, Sandkühler J. Gerhold KJ, et al. J Neurosci. 2015 Jul 1;35(26):9580-94. doi: 10.1523/JNEUROSCI.0731-14.2015. J Neurosci. 2015. PMID: 26134641 Free PMC article. - FDA Approved Drugs Repurposing of Toll-Like Receptor4 (TLR4) Candidate for Neuropathy.
Zali H, Golchin A, Farahani M, Yazdani M, Ranjbar MM, Dabbagh A. Zali H, et al. Iran J Pharm Res. 2019 Summer;18(3):1639-1647. doi: 10.22037/ijpr.2019.2394. Iran J Pharm Res. 2019. PMID: 32641971 Free PMC article. - Low-dose naltrexone rescues inflammation and insulin resistance associated with hyperinsulinemia.
Choubey A, Girdhar K, Kar AK, Kushwaha S, Yadav MK, Ghosh D, Mondal P. Choubey A, et al. J Biol Chem. 2020 Nov 27;295(48):16359-16369. doi: 10.1074/jbc.RA120.013484. Epub 2020 Sep 17. J Biol Chem. 2020. PMID: 32943552 Free PMC article.
References
- Ambriz-Tututi M, Granados-Soto V. Oral and spinal melatonin reduces tactile allodynia in rats via activation of MT2 and opioid receptors. Pain. 2007;132:273–280. - PubMed
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
- Chacur M, Milligan ED, Gazda LS, Armstrong C, Wang H, Tracey KJ, Maier SF, Watkins LR. A new model of sciatic inflammatory neuritis (SIN): induction of unilateral and bilateral mechanical allodynia following acute unilateral peri-sciatic immune activation in rats. Pain. 2001;94:231–244. - PubMed
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. - PubMed
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA015642/DA/NIDA NIH HHS/United States
- K02 DA015642/DA/NIDA NIH HHS/United States
- DA017670/DA/NIDA NIH HHS/United States
- K05 DA024044/DA/NIDA NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 DA017670/DA/NIDA NIH HHS/United States
- R01 DE017782/DE/NIDCR NIH HHS/United States
- DE017782/DE/NIDCR NIH HHS/United States
- R01 DA023132/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical