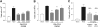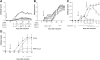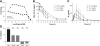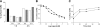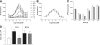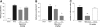An engineered monomer of CCL2 has anti-inflammatory properties emphasizing the importance of oligomerization for chemokine activity in vivo - PubMed (original) (raw)
doi: 10.1189/jlb.0108061. Epub 2008 Jul 28.
Zoë Johnson, David H Rodrigues, Adriana C Dos Santos, Rocco Cirillo, Valeria Muzio, Simona Riva, Matthias Mack, Maud Déruaz, Frédéric Borlat, Pierre-Alain Vitte, Timothy N C Wells, Mauro M Teixeira, Amanda E I Proudfoot
Affiliations
- PMID: 18662971
- PMCID: PMC2538597
- DOI: 10.1189/jlb.0108061
An engineered monomer of CCL2 has anti-inflammatory properties emphasizing the importance of oligomerization for chemokine activity in vivo
Tracy M Handel et al. J Leukoc Biol. 2008 Oct.
Abstract
We demonstrated recently that P8A-CCL2, a monomeric variant of the chemokine CCL2/MCP-1, is unable to induce cellular recruitment in vivo, despite full activity in vitro. Here, we show that this variant is able to inhibit CCL2 and thioglycollate-mediated recruitment of leukocytes into the peritoneal cavity and recruitment of cells into lungs of OVA-sensitized mice. This anti-inflammatory activity translated into a reduction of clinical score in the more complex inflammatory model of murine experimental autoimmune encephalomyelitis. Several hypotheses for the mechanism of action of P8A-CCL2 were tested. Plasma exposure following s.c. injection is similar for P8A-CCL2 and wild-type (WT) CCL2, ruling out the hypothesis that P8A-CCL2 disrupts the chemokine gradient through systemic exposure. P8A-CCL2 and WT induce CCR2 internalization in vitro and in vivo; CCR2 then recycles to the cell surface, but the cells remain refractory to chemotaxis in vitro for several hours. Although the response to P8A-CCL2 is similar to WT, this finding is novel and suggests that despite the presence of the receptor on the cell surface, coupling to the signaling machinery is retarded. In contrast to CCL2, P8A-CCL2 does not oligomerize on glycosaminoglycans (GAGs). However, it retains the ability to bind GAGs and displaces endogenous JE (murine MCP-1) from endothelial surfaces. Intravital microscopy studies indicate that P8A-CCL2 prevents leukocyte adhesion, while CCL2 has no effect, and this phenomenon may be related to the mechanism. These results suggest that oligomerization-deficient chemokines can exhibit anti-inflammatory properties in vivo and may represent new therapeutic modalities.
Figures
Fig. 1.
P8A-CCL2 inhibits cell recruitment in vivo. Data are expressed as mean total cell counts ±
sd
, and n = 5 mice per group. White bars indicate cellularity in unsensitized animals, maximal response with NaCl treatment is shown in black, and treatment with various doses of P8A-CCL2 is shown as gray bars. (A) Chemokine-induced peritoneal recruitment model: Cellular recruitment was induced by 10 μg CCL2 i.p., and the cells were enumerated following peritoneal lavage after 18 h as described in the text. P8A-CCL2 was administered i.p. 30 min prior to the CCL2 stimulus at the doses indicated. (B) Thioglycollate-induced recruitment model: Cellular recruitment was induced by thioglycollate into the peritoneal cavity as described in text. P8A-CCL2 was administered s.c. 15 min prior to the thioglycollate stimulus, and a second administration was given after 24 h. Cells were enumerated after 48 h. (C) OVA-sensitization model: Comparison of the inhibitory effect on cellular recruitment into the bronchiolar lavage (BAL) fluid by monomeric P8A-CCL2 (dark-gray bar) and [44AANA47]-CCL5 (light-gray bar) dosed at 0.5 mg/kg i.p. 30 min before challenge in OVA-induced airway inflammation. Inhibition is expressed as percent inhibition compared with the BAL cellularity of the NaCl-treated group as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
Fig. 2.
Treatment with P8A-CCL2 in the MOG-induced murine model of EAE reduces clinical score. (A) P8A-CCL2 was administered i.p. at Day 7 using the protocol with two MOG sensitizations as described in the text. (•) NaCl-treated control; (▵) 0.15 mg/kg; (○) 0.5 mg/kg; (□) 1.5 mg/kg. (B) P8A-CCL2 was administered i.p. at Day 7 (indicated by arrow) using the protocol with two MOG sensitizations as described in the text. (•) NaCl-treated control; (▵) 0.05 mg/kg; (○) 0.15 mg/kg; (□) 0.5 mg/kg; (C) (•) NaCl-treated control; P8A-CCL2 (1.5 mg/kg) was administered s.c. at Day 10 (□) and at Day 13 (○; indicated by arrow) using the protocol with a single MOG sensitization as described in the text. (D) (•) NaCl-treated control; (○) 1.5 mg /kg P8A-CCL2, and (▵) CCL2 were administered s.c. daily starting at Day 10 (indicated by arrow) using the protocol with a single MOG sensitization as described in the text. Significance is indicated by *, **, and *** for P < 0.05, P < 0.01, and P < 0.001, respectively, over the indicated period of days shown by the bars when compared with vehicle-treated animals.
Fig. 3.
P8A-CCL2 displaces murine JE from the endothelial surface. (A) The ability of CCL2 (•) and P8A-CCL2 (▪) to displace (in the case of P8A-CCL2) or increase (in the case of CCL2) the amount of [125I]-CCL2 onto heparin sepharose beads was determined in a competition equilibrium-binding assay. One of two experiments is shown. (B) P8A-CCL2 was administered s.c. at the doses indicated, and its concentration in the serum was determined by ELISA as described in the text at 0.5, 1, 2, 3, 4, 6, 8, 16, and 24 h. (C) The concentration of JE was determined by ELISA at the time-points indicated. (D) In a thioglycollate peritonitis model, a single dose of P8A-CCL2 (10 mg/kg) was given at the time-points indicated prior to the thioglycollate stimulus (gray bars). Dexamethasone (Dex; 1 mg/kg) was used as a positive control for inhibition of cell recruitment and administered s.c. 15 min prior to the thioglycollate stimulus (black bar). Cells were enumerated in the peritoneal cavity 48 h after the thioglycollate administration. The experiment was performed twice.
Fig. 4.
CCR2 down-regulation and recycling in vivo and in vitro. (A) Internalization of CCR2 on murine monocytes was determined by FACS analysis of GR1+ monocytes with an anti-mCCR2 antibody after i.p. administration of 0.5 and 3 mg/kg CCL2 (open bars) or P8A-CCL2 (gray bars). The surface expression of CCR2 prior to administration of the proteins is shown in the black bar. The results are representative of one of two experiments. (B) CCR2 down-regulation in vitro was determined by FACS analysis after incubation of purified GR1+ monocytes with CCL2 (•) or P8A-CCL2 (▪) at 37°C for 30 min. (C) Recycling of CCR2 as determined by FACS analysis after removal of CCL2 (•) or P8A-CCL2 (▪) from the culture supernatant. The results are representative of one of three experiments.
Fig. 5.
Chemotactic response following receptor recycling. (A) Induction of CCR2 down-modulation was induced in THP-1 cells with 0.1 μg P8A-CCL2, and the receptor was then allowed to recycle. Chemotaxis was determined as described in the text at the times indicated. The results are representative of one of two experiments. (B) The chemotactic response of THP-1 cells to CCL2 was determined at Time 0 (•) and after 24 h (○) in culture without prior stimulation. The results are representative of one of two experiments. (C) THP-1 cells were incubated with 0.1 μg/ml CCL2 (white bars) or P8A-CCL2 (gray bars) for 45 min at 37°C. The supernatant was removed to allow the receptor to recycle. Cell-surface receptor was determined by incubation of the cells with [125I]-CCL2 for 1 h as described in the text. The results are representative of one of two experiments. (D) P8A-CCL2 or saline control was administered i.p. 30 min prior to 0.5 mg/kg CCL2 or MCP-5, and the cells enumerated following peritoneal lavage as described in the text after 18 h. The white bar is the saline control; black bars represent the maximal response to CCL2 or MCP-5 using saline 30 min prior to CCL2 or MCP-5 administration; gray bars show the effect of administration of P8A-CCL2 30 min prior to CCL2/MCP-5 administration. Significance is indicated by ***, P < 0.001, when comparing with NaCl-treated animals.
Fig. 6.
P8A-CCL2 but not CCL2 prevents leukocyte adhesion in the brain microvasculature. Intravital microscopy was used to assess the rolling and firm arrest of leukocytes on pial microvessels. P8A-CCL2 (0.5 or 1.5 mg/kg, s.c.) was administered on Day 14 after EAE induction, and (A) leukocyte rolling and (B) adhesion on pial vessels were quantified after 45 min. Control animals received vehicle (200 μl saline). (C) Leukocyte adhesion was quantified 45 min after administration of P8A-CCL2 or CCL2 (both at 1.5 mg/kg, s.c.). The number of animals in each experimental group was ≥5, and results are shown as the mean ±
sem
. Significance is indicated by *, P < 0.05, and **, P < 0.01, when comparing with NaCl-treated animals.
Similar articles
- Multiple glycosaminoglycan-binding epitopes of monocyte chemoattractant protein-3/CCL7 enable it to function as a non-oligomerizing chemokine.
Salanga CL, Dyer DP, Kiselar JG, Gupta S, Chance MR, Handel TM. Salanga CL, et al. J Biol Chem. 2014 May 23;289(21):14896-912. doi: 10.1074/jbc.M114.547737. Epub 2014 Apr 11. J Biol Chem. 2014. PMID: 24727473 Free PMC article. - CCR2 expression by brain microvascular endothelial cells is critical for macrophage transendothelial migration in response to CCL2.
Dzenko KA, Song L, Ge S, Kuziel WA, Pachter JS. Dzenko KA, et al. Microvasc Res. 2005 Jul;70(1-2):53-64. doi: 10.1016/j.mvr.2005.04.005. Epub 2005 May 31. Microvasc Res. 2005. PMID: 15927208 - Administration of a monomeric CCL2 variant to EAE mice inhibits inflammatory cell recruitment and protects from demyelination and axonal loss.
Brini E, Ruffini F, Bergami A, Brambilla E, Dati G, Greco B, Cirillo R, Proudfoot AE, Comi G, Furlan R, Zaratin P, Martino G. Brini E, et al. J Neuroimmunol. 2009 Apr 30;209(1-2):33-9. doi: 10.1016/j.jneuroim.2009.01.022. Epub 2009 Feb 15. J Neuroimmunol. 2009. PMID: 19232440 - Multimerization of monocyte chemoattractant protein-1 is not required for glycosaminoglycan-dependent transendothelial chemotaxis.
Ali S, Palmer AC, Fritchley SJ, Maley Y, Kirby JA. Ali S, et al. Biochem J. 2001 Sep 15;358(Pt 3):737-45. doi: 10.1042/0264-6021:3580737. Biochem J. 2001. PMID: 11535134 Free PMC article. - The roles of chemokines in leukocyte recruitment and fibrosis in systemic sclerosis.
Hasegawa M, Sato S. Hasegawa M, et al. Front Biosci. 2008 May 1;13:3637-47. doi: 10.2741/2955. Front Biosci. 2008. PMID: 18508461 Review.
Cited by
- Heterodimers Are an Integral Component of Chemokine Signaling Repertoire.
Kaffashi K, Dréau D, Nesmelova IV. Kaffashi K, et al. Int J Mol Sci. 2023 Jul 19;24(14):11639. doi: 10.3390/ijms241411639. Int J Mol Sci. 2023. PMID: 37511398 Free PMC article. Review. - Disruption of monocyte-macrophage differentiation and trafficking by a heme analog during active inflammation.
Schaefer REM, Callahan RC, Atif SM, Orlicky DJ, Cartwright IM, Fontenot AP, Colgan SP, Onyiah JC. Schaefer REM, et al. Mucosal Immunol. 2022 Feb;15(2):244-256. doi: 10.1038/s41385-021-00474-8. Epub 2021 Dec 16. Mucosal Immunol. 2022. PMID: 34916594 Free PMC article. - Neutrophil Extracellular Traps Induce MCP-1 at the Culprit Site in ST-Segment Elevation Myocardial Infarction.
Hofbauer TM, Ondracek AS, Mangold A, Scherz T, Nechvile J, Seidl V, Brostjan C, Lang IM. Hofbauer TM, et al. Front Cell Dev Biol. 2020 Nov 9;8:564169. doi: 10.3389/fcell.2020.564169. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33240874 Free PMC article. - Targeting Chemokine-Glycosaminoglycan Interactions to Inhibit Inflammation.
Crijns H, Vanheule V, Proost P. Crijns H, et al. Front Immunol. 2020 Mar 31;11:483. doi: 10.3389/fimmu.2020.00483. eCollection 2020. Front Immunol. 2020. PMID: 32296423 Free PMC article. Review. - More Than Just Attractive: How CCL2 Influences Myeloid Cell Behavior Beyond Chemotaxis.
Gschwandtner M, Derler R, Midwood KS. Gschwandtner M, et al. Front Immunol. 2019 Dec 13;10:2759. doi: 10.3389/fimmu.2019.02759. eCollection 2019. Front Immunol. 2019. PMID: 31921102 Free PMC article. Review.
References
- Luster A D. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. - PubMed
- Handel T M, Johnson Z, Crown S E, Lau E K, Proudfoot A E. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. - PubMed
- Rollins B J. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. - PubMed
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous