ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia - PubMed (original) (raw)
ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia
Marion Faigle et al. PLoS One. 2008.
Abstract
Background: Extracellular ATP is an important signaling molecule for vascular adaptation to limited oxygen availability (hypoxia). Here, we pursued the contribution of vascular endothelia to extracellular ATP release under hypoxic conditions.
Methodology, principal findings: We gained first insight from studying ATP release from endothelia (HMEC-1) pre-exposed to hypoxia. Surprisingly, we found that ATP release was significantly attenuated following hypoxia exposure (2% oxygen, 22+/-3% after 48 h). In contrast, intracellular ATP was unchanged. Similarly, lactate-dehydrogenase release into the supernatants was similar between normoxic or hypoxic endothelia, suggesting that differences in lytic ATP release between normoxia or hypoxia are minimal. Next, we used pharmacological strategies to study potential mechanisms for endothelial-dependent ATP release (eg, verapamil, dipyridamole, 18-alpha-glycyrrhetinic acid, anandamide, connexin-mimetic peptides). These studies revealed that endothelial ATP release occurs--at least in part--through connexin 43 (Cx43) hemichannels. A real-time RT-PCR screen of endothelial connexin expression showed selective repression of Cx43 transcript and additional studies confirmed time-dependent Cx43 mRNA, total and surface protein repression during hypoxia. In addition, hypoxia resulted in Cx43-serine368 phosphorylation, which is known to switch Cx43 hemi-channels from an open to a closed state.
Conclusions/significance: Taken together, these studies implicate endothelial Cx43 in hypoxia-associated repression of endothelial ATP release.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
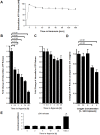
Figure 1. Endothelial ATP release during hypoxia.
A, To study extracellular ATP release from normoxic endothelia, monolayers of confluent HMEC-1 were washed and the culture media was replace with calcium containing HBSS. ATP content from their supernatant was sampled at indicated time points and quantified using a luminometric ATP detection assay. B, To measure extracellular ATP release under hypoxic conditions, confluent HMEC-1 monolayers were exposed to hypoxia (2% oxygen) over indicated time periods. Culture media was replaced with calcium containing HBSS and the ATP content within the supernatant was measured after 30 min incubation time. C, For intracellular ATP measurement, confluent HMEC-1 monolayers were exposed to hypoxia over indicated time periods, culture medium was discarded and cells were lysed by adding ice-cold water. ATP concentrations were measured as above. D, To measure the influence of different oxygen concentrations on endothelial ATP release, HMEC-1 were exposed to indicated degrees of hypoxia (21–2% of oxygen) over 24 h. Culture media was replaced with calcium containing HBSS and the ATP content within the supernatant was measured after 30 min incubation time. E, Confluent HMEC-1 monolayers were exposed to hypoxia as indicated. To assess lytic ATP release, LDH concentrations within the supernatant were measured by an LDH detection kit. In control experiments, cells were lysed with Triton X-100 (*p<0.01, n = 6 for all experiments).
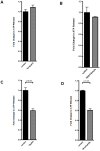
Figure 2. Molecular mechanisms of endothelial-dependent ATP release.
A–D, Confluent HMEC-1 monolayers were washed and exposed to Verapamil (10 µmol) or Dipyridamole (1 µmol) over 20 min, 18-alpha-glycyrrhetinic acid (18αGA, 20 µmol) or Anandamide (40 µmol) over 10 min. ATP content within the supernatant was measured by a luminometric ATP detection assay and compared with untreated control cells (n = 6).
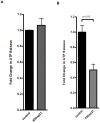
Figure 3. Connexin-mimetic peptides in endothelial ATP release.
A, B, Confluent HMEC-1 monolayers were washed and treated with connexin-mimetic peptides (A: Cx40 peptide, SRPTEKNVFIV, 50 µmol; B: Cx43 peptide, SRPTEKTIFII, 50 µmol). ATP content within the supernatant was measured by a luminometric ATP detection assay after an incubation period of 20 min and compared with control HMEC-1 treated with 50 µM bovine albumin (n = 6).
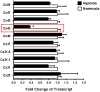
Figure 4. Endothelial connexin expression.
Confluent HMEC-1 monolayers were exposed to normoxia or hypoxia (12 h). Total RNA was isolated and real-time reverse-transcriptase polymerase chain reaction was employed to screen for transcriptional modulation of connexin expression. Data were calculated relative to an internal control gene (β-actin) and are expressed as fold change over normoxia at each time point. Results are derived from 3 experiments in each condition.
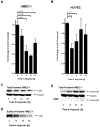
Figure 5. Influence of hypoxia on Connexin 43 expression.
A, B. Confluent HMEC-1 or HUVEC monolayers were exposed to normoxia or hypoxia (2% oxygen) over indicated time periods. Total RNA was isolated and transcriptional responses were assessed by real-time reverse-transcriptase polymerase chain reaction. Data were calculated relative to an internal housekeeping gene (β-actin) and are expressed as fold change over normoxia at each time point. Results are derived from 3 experiments in each condition. C. Confluent HMEC-1 monolayers were exposed to hypoxia over indicated time periods. Cells were lysed and proteins were resolved by SDS-PAGE and transferred to PVDF-membrane. Membranes were probed with a connexin 43 antibody, proteins were detected by chemiluminescene. The same blot was reprobed for β-actin as a control for protein loading. A representative experiment of 3 is shown. D. Confluent HMEC-1 monolayers were exposed to hypoxia over indicated time periods. Monolayers were washed, surface proteins were biotinylated, and cells were lysed. Connexin 43 was immunoprecipitated, followed by addition of Protein G Microbeads. Proteins were resolved by SDS-PAGE and resultant Western blots were probed with Streptavidin. A representative experiment of 3 is shown. E. Confluent HMEC-1 monolayers were exposed to normoxia or hypoxia over indicated time periods. Cells were lysed and proteins were resolved by SDS-PAGE and transferred to PVDF-Membrane. Membranes were probed with phospho-connexin 43 antibody specific for phosphorylated ser368, and proteins were detected by chemiluminescene. The same blot was probed for β-actin as a control for protein loading. A representative of 3 is shown. In subsets of experiments, cells were pretreated with the protein kinase C inhibitor bisindolylmaleimide (10 µM; +BIM).
Figure 6. ATP release from HMEC treated with the protein kinase C inhibitor bisindolylmaleimide (BIM).
To study the role of Cx43 ser398 phosphorylation status in ATP release from endothelia, monolayers of confluent HMEC-1 were treated with BIM (+BIM, 10 µM) or vehicle control (-BIM), exposed to normoxia or hypoxia (24 h, 2% oxygen), washed and the culture media was replace with calcium containing HBSS. ATP content from the supernatant was sampled at indicated time points and quantified using a luminometric ATP detection assay (*p<0.05 compared to Normoxia – BIM; n = 6).
Similar articles
- ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function.
Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP. Eltzschig HK, et al. Circ Res. 2006 Nov 10;99(10):1100-8. doi: 10.1161/01.RES.0000250174.31269.70. Epub 2006 Oct 12. Circ Res. 2006. PMID: 17038639 - Inhibition of connexin 43 hemichannel-mediated ATP release attenuates early inflammation during the foreign body response.
Calder BW, Matthew Rhett J, Bainbridge H, Fann SA, Gourdie RG, Yost MJ. Calder BW, et al. Tissue Eng Part A. 2015 Jun;21(11-12):1752-62. doi: 10.1089/ten.TEA.2014.0651. Epub 2015 Mar 26. Tissue Eng Part A. 2015. PMID: 25760687 Free PMC article. - Association Between Adenosine A2A Receptors and Connexin 43 Regulates Hemichannels Activity and ATP Release in Astrocytes Exposed to Amyloid-β Peptides.
Madeira D, Dias L, Santos P, Cunha RA, Canas PM, Agostinho P. Madeira D, et al. Mol Neurobiol. 2021 Dec;58(12):6232-6248. doi: 10.1007/s12035-021-02538-z. Epub 2021 Sep 2. Mol Neurobiol. 2021. PMID: 34476674 - Connexin and pannexin hemichannels in inflammatory responses of glia and neurons.
Bennett MV, Garré JM, Orellana JA, Bukauskas FF, Nedergaard M, Sáez JC. Bennett MV, et al. Brain Res. 2012 Dec 3;1487:3-15. doi: 10.1016/j.brainres.2012.08.042. Epub 2012 Sep 10. Brain Res. 2012. PMID: 22975435 Free PMC article. Review. - Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels.
Iyyathurai J, D'hondt C, Wang N, De Bock M, Himpens B, Retamal MA, Stehberg J, Leybaert L, Bultynck G. Iyyathurai J, et al. Neuropharmacology. 2013 Dec;75:491-505. doi: 10.1016/j.neuropharm.2013.04.050. Epub 2013 May 7. Neuropharmacology. 2013. PMID: 23664811 Review.
Cited by
- Gap junctions.
Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Nielsen MS, et al. Compr Physiol. 2012 Jul;2(3):1981-2035. doi: 10.1002/cphy.c110051. Compr Physiol. 2012. PMID: 23723031 Free PMC article. Review. - Signaling through hepatocellular A2B adenosine receptors dampens ischemia and reperfusion injury of the liver.
Zimmerman MA, Grenz A, Tak E, Kaplan M, Ridyard D, Brodsky KS, Mandell MS, Kam I, Eltzschig HK. Zimmerman MA, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):12012-7. doi: 10.1073/pnas.1221733110. Epub 2013 Jun 28. Proc Natl Acad Sci U S A. 2013. PMID: 23812746 Free PMC article. Retracted. - The Role of Connexin Hemichannels in Inflammatory Diseases.
Peng B, Xu C, Wang S, Zhang Y, Li W. Peng B, et al. Biology (Basel). 2022 Feb 2;11(2):237. doi: 10.3390/biology11020237. Biology (Basel). 2022. PMID: 35205103 Free PMC article. Review. - Sphingosine-1-phosphate receptor signaling during acute kidney injury: the tissue is the issue.
Bartels K, Grenz A, Eltzschig HK. Bartels K, et al. Kidney Int. 2014 Apr;85(4):733-5. doi: 10.1038/ki.2013.435. Kidney Int. 2014. PMID: 24682118 Free PMC article. - Extracellular ATP: A Feasible Target for Cancer Therapy.
Vultaggio-Poma V, Sarti AC, Di Virgilio F. Vultaggio-Poma V, et al. Cells. 2020 Nov 17;9(11):2496. doi: 10.3390/cells9112496. Cells. 2020. PMID: 33212982 Free PMC article. Review.
References
- Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14:1315–1323. - PubMed
- Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annual Review of Immunology. 2004;22:657–682. - PubMed
- Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. - PubMed
- Van Linden A, Eltzschig HK. Role of pulmonary adenosine during hypoxia: extracellular generation, signaling and metabolism by surface adenosine deaminase/CD26. Expert Opin Biol Ther. 2007;7:1437–1447. - PubMed
- Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases