Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels - PubMed (original) (raw)
Dual orexin actions on dorsal raphe and laterodorsal tegmentum neurons: noisy cation current activation and selective enhancement of Ca2+ transients mediated by L-type calcium channels
K A Kohlmeier et al. J Neurophysiol. 2008 Oct.
Abstract
The hypocretin/orexins (Hcrt/Orxs) are hypothalamic neuropeptides that regulate stress, addiction, feeding, and arousal behaviors. They depolarize many types of central neurons and can increase [Ca2+]i in some, including those of the dorsal raphe (DR) and laterodorsal tegmental (LDT) nuclei-two structures likely to contribute to the behavioral actions of Hcrt/Orx. In this study, we used simultaneous whole cell and Ca2+-imaging methods in mouse brain slices to compare the Hcrt/Orx-activated current in DR and LDT neurons and to determine whether it contributes to the Ca2+ influx evoked by Hcrt/Orx. We found Hcrt/Orx activates a similar noisy cation current that reversed near 0 mV in both cell types. Contrary to our expectation, this current did not contribute to the somatic Ca2+ influx evoked by Hcrt/Orx. In contrast, Hcrt/Orx enhanced the Ca2+ transients produced by voltage steps (-60 to -30 mV) by approximately 30% even in neurons lacking an inward current. This effect was abolished by nifedipine, augmented by Bay-K and abolished by bisindolylmaleimide I. Thus Hcrt/Orx has two independent actions: activation of noisy cation channels that generate depolarization and activation of a protein kinase C (PKC)-dependent enhancement of Ca2+ transients mediated by L-type Ca2+ channels. Immunocytochemistry verified that both these actions occurred in serotonergic and cholinergic neurons, indicating that Hcrt/Orx can function as a neuromodulator in these key neurons of the reticular activating system. Because regulation of Ca2+ transients mediated by L-channels is often linked to the control of transcriptional signaling, our findings imply that Hcrt/Orxs may also function in the regulation of long-term homeostatic or trophic processes.
Figures
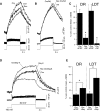
FIG. 1.
Hypocretin/orexin (Hcrt/Orx) depolarizes dorsal raphe (DR) and laterodorsal tegmental (LDT) neurons causing action potentials and rises in somatic and proximal dendritic [Ca2+]i. A and B: (top right) images of bis-fura-2 fluorescence in a DR (A) and LDT (B) neuron during whole cell recordings from which changes in fluorescence and membrane potential were measured (left column). Boxes indicate regions of interest (ROIs) from which dF/F signals were computed. Scale equals 10 μm. In left panels, bottom trace indicates membrane potential, middle trace indicates somatic dF/F, and top trace indicates dendritic dF/F. Orexin-A (300 nM) induced a depolarization, firing, and rises in [Ca2+]i in these DR and LDT neurons. Boxed insets are high-gain traces showing that the Ca2+ signals preceded the induction of spiking (arrows). Scales in insets equal 10% dF/F, 10 mV, and 20 s. C: immunocytochemistry verified that these actions occurred in serotonergic and cholinergic neurons of the DR (C1) and LDT (C2). Left image was taken at low power (×4 objective) and shows TpH-positive cells in the Raphe (C1) and bNOS-positive cells in the LDT (C2) labeled with FITC. Top image in right columns show a higher-power FITC image (×20 objective) of the field containing the recorded neuron. Bottom image in right columns shows the recorded neuron (arrow) in the DR (C1) and the LDT (C2) visualized with alexa-594, which was included in the pipette solution. These neurons showed an orexin-A (300 nM)–mediated depolarization and change in [Ca2+]I and were immunopositive for TpH (C1) and hence serotonergic and bNOS (C2) and hence cholinergic. Scale equals 100 (×4) and 20 μm (×20). Image brightness and contrast were adjusted uniformly for each image to facilitate viewing fluorescent labels. Aq, aqueduct; MnR, median raphe; DTg, dorsal tegmental nucleus.
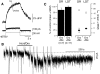
FIG. 2.
Orexin activates an Na+-dependent cation current that is similar in DR (A–G) and LDT neurons (H–L). A: bath application of orexin-A (300 nM) produces a long-lasting inward current (bottom trace) and increase in current noise (top trace) in DR neurons (holding potential = –60 mV). B: following activation of this current by orexin-A, superfusion with low-Na+ artificial cerebrospinal fluid (ACSF) rapidly attenuated the current and the noise increase, consistent with a large reduction in concentration of a permeant ion. Return to ACSF containing normal Na+ reinstated a residual noisy inward current. C: application of low Na+ ACSF also attenuated the baseline holding current and noise (−60 mV), showing that the basal inward current at −60 mV is also Na+ dependent. D: application of orexin-A (300 nM) under low Na+ conditions produced only a small inward current in DR neurons. E: Voltage ramps between –100 and –30 mV obtained before and after orexin application showed an inward current that was larger and noisier at –100 than at –30 mV. F: I-V relation of the orexin-evoked current (bottom) appeared quite linear over this range as indicated by the best fit line. The noise also decreased linearly between –100 and –30 mV (top). G: the orexin current appeared similar in recordings obtained with Cs+-rich intracellular solutions and reversed near 0 mV, suggesting the orexin-activated channels were permeable to both Cs+ and K+. H: in LDT neurons, bath application of orexin-A (300 nM) also produced a long-lasting inward current (bottom trace) and an increase in membrane current noise (top trace). I: both were rapidly attenuated by low-Na+ ACSF. J: voltage ramps (–100 to –30 mV) often showed a noisy current similar to that seen in DR neurons. K: in these LDT neurons, the I-V relation of the orexin-evoked current was linear over this range as indicated by the best-fit line (bottom). The membrane current noise also decreased between –100 and –30 mV (top). L: this orexin current appeared similar in LDT neurons recorded with Cs+-rich intracellular solutions and reversed near 0 mV, suggesting the underlying channels are permeable to Cs+ and K+ as in DR neurons. For experiments A–F and H–K, the internal solution contained K+ gluconate and the ACSF contained DABST and 2 mM Cs+. For experiments G and L, the internal solution contained Cs+ gluconate and the ACSF contained low Ca2+, DABST, and 2 mM Cs+.
FIG. 3.
Orexin-A causes rises in [Ca2+]i and Ca2+ spiking in some DR and LDT neurons, if they are sufficiently depolarized. A: following application of TTX and the blockade of ionotropic glutamate, GABA, and glycine receptors, orexin-A often elicited Ca2+ spikes in DR (left traces) and LDT (middle traces) neurons. Right traces show the Ca2+ spikes obtained by depolarization in the DABST-containing ACSF and the spikes obtained in normal ACSF. Note the difference in threshold and amplitude. B: changes in [Ca2+]i were also elicited by orexin-A in some cells in which Ca2+ spikes were not induced. C: in some cells, the orexin-A–induced depolarization was not accompanied by changes in dF/F. Note: the small change in dF/F during the application is artifact, often seen with the “Y-tube” method of drug delivery, and was easily distinguished from specific peptide effects. The bar graph shows the maximal percent change in dF/F produced by orexin-A (300 nM) in cells that were held close to −45 mV and those that were held close to −55 mV. The holding potential influenced the change in [Ca2+]i induced by the Hcrt/Orx depolarization. D: depolarization elicited by a simulated Hcrt/Orx current induced rises in [Ca2+]i. The current command was derived from a previously recorded inward current induced by orexin-A. This current command produced a slow depolarization that was sufficient to induce an increase in dF/F in the absence of Hcrt/Orx in this DR cell at a baseline potential of –43 mV (D1, left). The same current command elicited a negligible change in dF/F when injected from a baseline potential of –60 mV in the same cell (D1, right). Similarly, at a given baseline potential, increasing the injected current, increased the resulting dF/F (D2; LDT neuron).
FIG. 4.
The nonselective cation channel (NSCC) activated by Hcrt/Orx is not a Ca2+ influx pathway that contributes to the Ca2+ transients elicited by Hcrt/Orx. A: voltage-clamp recordings (holding = −60 mV) and corresponding changes in dF/F following Y-tube application of ACSF (1) and orexin-A in ACSF (2) to a DR (left traces) and LDT (right traces) neuron. Orexin-A induced an inward current but no rise in somatic or dendritic [Ca2+]i when DR and LDT neurons were voltage clamped near rest (−60 mV). B: voltage jumps to –100 mV increased the driving force for Ca2+ and augmented the dF/F under baseline conditions, indicating a resting Ca2+ permeability. However, this increase in dF/F was not further augmented during activation of the NSCC by orexin-A. B1: membrane current (holding = –60 mV) recorded from a DR neurons while orexin-A (300 nM) was applied. The trace was “blanked” during voltage jumps before (B1a) and after (B1b) orexin-A application. B2a: dF/F (top trace) increased during voltage steps from –60 to –100 mV (bottom traces), indicating a resting Ca2+ permeability (3 sweeps superimposed; middle trace is membrane current). B2b: This slight increase in dF/F was not different when tested during the inward current elicited by orexin-A application (3 traces superimposed). B3: bar graph summarizes these changes in dF/F elicited by voltage jumps to −100 mV from before and after orexin-A application. These changes were not different (P > 0.05, n = 5), indicating that any Ca2+ influx that might occur via the NSCC does not contribute directly to the Hcrt/Orx-mediated Ca2+ transients.
FIG. 5.
Hcrt/Orx enhances the Ca2+ transients produced by activation of L-type Ca2+ channels in DR and LDT neurons. A: Ca2+ transients elicited by voltage steps from –60 to –30 mV were reversibly enhanced by ∼30% following application of orexin-A (300 nM) in DR and LDT neurons, even in the absence of an orexin-A–induced inward current. Top traces show the Ca2+ transients measured as dF/F superimposed from before orexin-A application (Con), after orexin-A application (Orx), and after recovery from the orexin-A application (Rec). The bottom and middle traces show the corresponding membrane voltage (bottom) and membrane current traces (middle). B: nifedipine (10 μM) nearly completely attenuated the Ca2+ transient enhancement produced by Hcrt/Orx. Top traces show the Ca2+ transients elicited by voltage steps from –60 to –30 mV in the presence of nifedipine (Nif) after orexin-A application (Orx/Nif) and after the expected orexin effect would have recovered (Rec Orx/Nif). C: bar graph summarizes the effect of nifedipine (10 μM) on the Ca2+ transient enhancement produced by Hcrt/Orx. Nifedipine significantly attenuated this effect in both the DR and LDT (*P < 0.05). D: application of Bay-K 8644 (10 μm; Bay-K), which enhances current through L-type Ca2+ channels, increased the control Ca2+ transients elicited by voltage steps from –60 to –30 mV (Con). Application of orexin-A (300 nM) further enhanced these Ca2+ transients (Orx/Bay-K), which recovered after wash-out of orexin-A (Rec Orx/Bay-K). E: bar graph comparing the enhancement of the voltage step–evoked Ca2+ transient produced by orexin-A in normal ACSF (Orx ACSF) and in ACSF containing Bay K (Orx + Bay). The Ca2+ transient enhancement was significantly larger in the presence of Bay-K than it was in the absence of Bay-K for both DR and LDT neurons (*P < 0.05).
FIG. 6.
Inhibition of protein kinase C (PKC) with bisindolylmaleimide I abolishes enhancement of voltage-step evoked Ca2+ transients by Hcrt/Orx but does not completely block the Hcrt/Orx-evoked inward currents. A: Ca2+ transients elicited in DR and LDT neurons by voltage-steps from –60 to –30 mV following preincubation with bisindolylmaleimide I (Bis I; top trace labeled bis) were not enhanced by orexin-A (300 nM; top trace labeled Orx/bis). Corresponding voltage (bottom) and total membrane current (middle) traces are also shown. B: following preincubation with Bis I, orexin-A (300 nM) retained the ability to evoke an inward current in some neurons even though these neurons did not show an enhancement in Ca2+ transients evoked by voltage steps. Trace in B is taken from 1 such neuron recorded in the DR. Note that the vertical deflections are currents associated with test voltage steps and are truncated. C: left bar graph: effect of Hcrt/Orx on Ca2+ transients following preincubation with Bis I. The average Ca2+ transient amplitude was not different following orexin-A application. Right graph: magnitude of the current evoked by orexin-A following preincubation of the slice with Bis I in the same neurons that were imaged. There was no detectable current in 5/7 DR neurons and 9/11 LDT neurons. The average (±SE) Hcrt/Orx current for these neurons is plotted as solid symbols for DR and LDT neurons (_I_Orx). Two neurons from each nucleus, however, retained an _I_Orx of apparently normal magnitude. The amplitudes of these currents are plotted as open symbols for each DR and LDT neuron.
Similar articles
- Hypocretin/orexin peptide signaling in the ascending arousal system: elevation of intracellular calcium in the mouse dorsal raphe and laterodorsal tegmentum.
Kohlmeier KA, Inoue T, Leonard CS. Kohlmeier KA, et al. J Neurophysiol. 2004 Jul;92(1):221-35. doi: 10.1152/jn.00076.2004. Epub 2004 Mar 3. J Neurophysiol. 2004. PMID: 14999052 - Direct and indirect excitation of laterodorsal tegmental neurons by Hypocretin/Orexin peptides: implications for wakefulness and narcolepsy.
Burlet S, Tyler CJ, Leonard CS. Burlet S, et al. J Neurosci. 2002 Apr 1;22(7):2862-72. doi: 10.1523/JNEUROSCI.22-07-02862.2002. J Neurosci. 2002. PMID: 11923451 Free PMC article. - Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions.
Liu RJ, van den Pol AN, Aghajanian GK. Liu RJ, et al. J Neurosci. 2002 Nov 1;22(21):9453-64. doi: 10.1523/JNEUROSCI.22-21-09453.2002. J Neurosci. 2002. PMID: 12417670 Free PMC article. - Acid-sensing hypothalamic neurons controlling arousal.
Kernder A, De Luca R, Yanovsky Y, Haas HL, Sergeeva OA. Kernder A, et al. Cell Mol Neurobiol. 2014 Aug;34(6):777-89. doi: 10.1007/s10571-014-0065-6. Epub 2014 May 6. Cell Mol Neurobiol. 2014. PMID: 24798513 Free PMC article. Review. - From Molecule to Behavior: Hypocretin/orexin Revisited From a Sex-dependent Perspective.
Gao XB, Horvath TL. Gao XB, et al. Endocr Rev. 2022 Jul 13;43(4):743-760. doi: 10.1210/endrev/bnab042. Endocr Rev. 2022. PMID: 34792130 Free PMC article. Review.
Cited by
- The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions.
Inutsuka A, Yamanaka A. Inutsuka A, et al. Front Endocrinol (Lausanne). 2013 Mar 6;4:18. doi: 10.3389/fendo.2013.00018. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23508038 Free PMC article. - Hypothalamic orexinergic neuron changes during the hibernation of the Syrian hamster.
López JM, Carballeira P, Pozo J, León-Espinosa G, Muñoz A. López JM, et al. Front Neuroanat. 2022 Sep 9;16:993421. doi: 10.3389/fnana.2022.993421. eCollection 2022. Front Neuroanat. 2022. PMID: 36157325 Free PMC article. - Orexin receptors 1 and 2 in serotonergic neurons differentially regulate peripheral glucose metabolism in obesity.
Xiao X, Yeghiazaryan G, Hess S, Klemm P, Sieben A, Kleinridders A, Morgan DA, Wunderlich FT, Rahmouni K, Kong D, Scammell TE, Lowell BB, Kloppenburg P, Brüning JC, Hausen AC. Xiao X, et al. Nat Commun. 2021 Sep 2;12(1):5249. doi: 10.1038/s41467-021-25380-2. Nat Commun. 2021. PMID: 34475397 Free PMC article. - Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats.
Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA. Lungwitz EA, et al. Physiol Behav. 2012 Dec 5;107(5):726-32. doi: 10.1016/j.physbeh.2012.05.019. Epub 2012 May 28. Physiol Behav. 2012. PMID: 22652097 Free PMC article. - Control of sleep and wakefulness.
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Brown RE, et al. Physiol Rev. 2012 Jul;92(3):1087-187. doi: 10.1152/physrev.00032.2011. Physiol Rev. 2012. PMID: 22811426 Free PMC article. Review.
References
- Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Muhlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience 142: 999–1004, 2006. - PubMed
- Brown RE, Sergeeva O, Eriksson KS, Haas HL. Orexin A excites serotonergic neurons in the dorsal raphe nucleus of the rat. Neuropharmacology 40: 457–459, 2001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous