GDPD5 is a glycerophosphocholine phosphodiesterase that osmotically regulates the osmoprotective organic osmolyte GPC - PubMed (original) (raw)
Comparative Study
. 2008 Aug 5;105(31):11026-31.
doi: 10.1073/pnas.0805496105. Epub 2008 Jul 30.
Affiliations
- PMID: 18667693
- PMCID: PMC2504799
- DOI: 10.1073/pnas.0805496105
Comparative Study
GDPD5 is a glycerophosphocholine phosphodiesterase that osmotically regulates the osmoprotective organic osmolyte GPC
Morgan Gallazzini et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Glycerophosphocholine (GPC) is an abundant osmoprotective renal medullary organic osmolyte. We previously found that its synthesis from phosphatidylcholine is catalyzed by tonicity-regulated activity of the phospholipase B, neuropathy target esterase. We also found that its degradation is catalyzed by glycerophosphocholine phosphodiesterase (GPC-PDE) activity and that elevating osmolality from 300 to 500 mosmol/kg by adding NaCl or urea, inhibits GPC-PDE activity, which contributes to the resultant increase of GPC. In the present studies we identify GDPD5 (glycerophosphodiester phosphodiesterase domain containing 5) as a GPC-PDE that is rapidly inhibited by high NaCl or urea. Recombinant GDPD5 colocalizes with neuropathy target esterase in the perinuclear region of HEK293 cells, and immuno-precipitated recombinant GDPD5 degrades GPC in vitro. The in vitro activity is reduced when the cells from which the GDPD5 is immuno-precipitated have been exposed to high NaCl or urea. In addition, high NaCl decreases mRNA abundance of GDPD5 via an increase of its degradation rate, although high urea does not. At 300 mosmol/kg siRNA knockdown of GDPD5 increases GPC in mouse inner medullary collecting duct-3 cells, and over expression of recombinant GDPD5 increases cellular GPC-PDE activity, accompanied by decreased GPC. We conclude that GDPD5 is a GPC-PDE that contributes to osmotic regulation of cellular GPC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
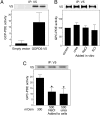
Fig. 1.
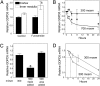
GDPD5 mRNA abundance in vivo and in mIMDC3 cells. (A) Effect of furosemide on GDPD5 mRNA abundance in mouse renal cortex and medulla. Kidneys were removed for analysis 4 h after i.p. injection of 1.5 mg of furosemide per 20 g of body weight. (B) Effect of hyperosmolality in mIMCD3 cells relative to the initial value at 300 mosmol/kg. (C) Effect on mIMCD3 cells of elevating NaCl or urea for 24 h, relative to the initial value at 300 mosmol/kg. (D) Effect on GDPD5 mRNA stability mIMCD3 cells of increasing osmolality from 300 to 500 mosmol/kg by adding NaCl. Five μg/ml actinomycin D was added 2 h after increasing NaCl. mRNA abundance was measured by real-time RT-PCR. (Mean ± SEM, n = 3, *, P < 0.05).
Fig. 2.
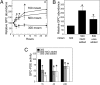
Effect of hyperosmolality on GPC abundance and GPC-PDE activity in mIMCD3 cells. (A) Effects of increasing osmolality on GPC abundance (percentage of the initial value of 17.6 ± 0.6 nmoles/mg protein at 300 mosmol/kg). (B) Effects on GPC abundance of increasing NaCl or urea for 24 h. GPC abundance is expressed as percentage of the initial value at 300 mosmol/kg. (C) Effect on GPC-PDE activity (expressed as percentage of the initial value at 300 mosmol/kg) of increasing NaCl or urea. (Mean ± SEM, n = 3, *, P < 0.05).
Fig. 3.
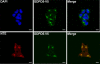
Subcellular localization of GDPD5. HEK293 cells were transfected with GDPD5-V5 at 300-mosmol/kg medium for 48 h, then fixed to visualize GDPD5-V5. Top panels: GDPD5-V5 (green) is localized in the perinuclear region. Nuclei are stained with DAPI (blue). Bottom panels: colocalization between GDPD5-V5 (green) and NTE (red) in the perinuclear region. (Scale bar = 20 μm.)
Fig. 4.
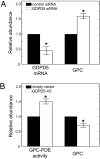
Effects of GDPD5 expression in mIMCD3 cells. (A) GDPD5 knock down with a specific siRNA at 300 mosmol/kg. The cells were transfected for 48 h with either control siRNA or GDPD5-specific siRNA. Left bars: Relative GDPD5 mRNA abundance. Right bars: Relative GPC abundance. (B) The cells were transfected with either empty vector or GDPD5-V5 construct at 300 mosmol/kg for 24 h. Left bars: Relative GPC abundance. Right bars: Relative GPC-PDE activity normalized by the total protein per dish of cells. (Mean ± SEM, n = 3, *, P < 0.05).
Fig. 5.
GPC-PDE activity of GDPD5 immuno-precipitated from HEK293 cells and assayed in vitro. HEK293 cells were transfected for 24 h at 300 mosmol/kg with empty vector-V5 or GDPD5-V5. GPC-PDE activity was measured in immuno-precipitates from cell extracts and is normalized to the amount of extracted protein used for the immuno-precipitation. GDPD5-V5 expression was measured in the immuno-precipitates. Top panels are representative Western blots showing equivalent expression of GDPD5-V5 protein. (A) GPC-PDE activity is present in immuno-precipitates of GDPD5-V5, but not of empty vector-V5. (B) GDPD5 was immuno-precipitated from cells at 300 mosmol/kg, and its GPC-PDE activity measured in vitro, using the default buffer or buffers to which 200 mosmol/kg of NaCl, KCl, or urea was added. (C) After transfection, cells were incubated for 1 h either at 300 mosmol/kg or at 500 mosmol/kg (200-mosmol/kg NaCl or urea added). Note that GPC-PDE activity measured under identical conditions in vitro varies with the osmolality to which the cells had been exposed. (Mean ± SEM, n = 3, *, P < 0.05).
Similar articles
- High NaCl- and urea-induced posttranslational modifications that increase glycerophosphocholine by inhibiting GDPD5 phosphodiesterase.
Topanurak S, Ferraris JD, Li J, Izumi Y, Williams CK, Gucek M, Wang G, Zhou X, Burg MB. Topanurak S, et al. Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7482-7. doi: 10.1073/pnas.1305220110. Epub 2013 Apr 15. Proc Natl Acad Sci U S A. 2013. PMID: 23589856 Free PMC article. - Neuropathy target esterase catalyzes osmoprotective renal synthesis of glycerophosphocholine in response to high NaCl.
Gallazzini M, Ferraris JD, Kunin M, Morris RG, Burg MB. Gallazzini M, et al. Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15260-5. doi: 10.1073/pnas.0607133103. Epub 2006 Oct 2. Proc Natl Acad Sci U S A. 2006. PMID: 17015841 Free PMC article. - Betaine and inositol reduce MDCK cell glycerophosphocholine by stimulating its degradation.
Kwon ED, Zablocki K, Peters EM, Jung KY, García-Pérez A, Burg MB. Kwon ED, et al. Am J Physiol. 1996 Jan;270(1 Pt 1):C200-7. doi: 10.1152/ajpcell.1996.270.1.C200. Am J Physiol. 1996. PMID: 8772445 - What's new about osmotic regulation of glycerophosphocholine.
Gallazzini M, Burg MB. Gallazzini M, et al. Physiology (Bethesda). 2009 Aug;24:245-9. doi: 10.1152/physiol.00009.2009. Physiology (Bethesda). 2009. PMID: 19675355 Free PMC article. Review. - Molecular basis of osmotic regulation.
Burg MB. Burg MB. Am J Physiol. 1995 Jun;268(6 Pt 2):F983-96. doi: 10.1152/ajprenal.1995.268.6.F983. Am J Physiol. 1995. PMID: 7611465 Review.
Cited by
- Expression and Characterization of a Novel Glycerophosphodiester Phosphodiesterase from Pyrococcus furiosus DSM 3638 That Possesses Lysophospholipase D Activity.
Wang F, Lai L, Liu Y, Yang B, Wang Y. Wang F, et al. Int J Mol Sci. 2016 May 30;17(6):831. doi: 10.3390/ijms17060831. Int J Mol Sci. 2016. PMID: 27248999 Free PMC article. - Phosphatidylcholine and the CDP-choline cycle.
Fagone P, Jackowski S. Fagone P, et al. Biochim Biophys Acta. 2013 Mar;1831(3):523-32. doi: 10.1016/j.bbalip.2012.09.009. Epub 2012 Sep 23. Biochim Biophys Acta. 2013. PMID: 23010477 Free PMC article. Review. - Transcriptome reprogramming during severe dehydration contributes to physiological and metabolic changes in the resurrection plant Haberlea rhodopensis.
Liu J, Moyankova D, Lin CT, Mladenov P, Sun RZ, Djilianov D, Deng X. Liu J, et al. BMC Plant Biol. 2018 Dec 13;18(1):351. doi: 10.1186/s12870-018-1566-0. BMC Plant Biol. 2018. PMID: 30541446 Free PMC article. - GDE5 inhibition accumulates intracellular glycerophosphocholine and suppresses adipogenesis at a mitotic clonal expansion stage.
Okazaki Y, Nakamura K, Takeda S, Yoshizawa I, Yoshida F, Ohshima N, Izumi T, Klein JD, Kumrungsee T, Sands JM, Yanaka N. Okazaki Y, et al. Am J Physiol Cell Physiol. 2019 Feb 1;316(2):C162-C174. doi: 10.1152/ajpcell.00305.2018. Epub 2018 Nov 21. Am J Physiol Cell Physiol. 2019. PMID: 30462540 Free PMC article. - Human GDPD3 overexpression promotes liver steatosis by increasing lysophosphatidic acid production and fatty acid uptake.
Key CC, Bishop AC, Wang X, Zhao Q, Chen GY, Quinn MA, Zhu X, Zhang Q, Parks JS. Key CC, et al. J Lipid Res. 2020 Jul;61(7):1075-1086. doi: 10.1194/jlr.RA120000760. Epub 2020 May 19. J Lipid Res. 2020. PMID: 32430316 Free PMC article.
References
- Burg MB, Ferraris JD, Dmitireva NI. Cellular response to hyperosmotic stresses. Physiol. Rev. 2007;87:1441–1474. - PubMed
- Bauernschmitt HG, Kinne RK. Metabolism of the ‘organic osmolyte’ glycerophosphorylcholine in isolated rat inner medullary collecting duct cells. I. Pathways for synthesis and degradation. Biochim Biophys Acta. 1993;1148:331–341. - PubMed
- Bauernschmitt HG, Kinne RK. Metabolism of the ‘organic osmolyte’ glycerophosphorylcholine in isolated rat inner medullary collecting duct cells. II. Regulation by extracellular osmolality. Biochim Biophys Acta. 1993;1150:25–34. - PubMed
- Kwon ED, Jung KY, Edsall LC, Kim HY, Garcia-Perez A, Burg MB. Osmotic regulation of synthesis of glycerophosphocholine from phosphatidylcholine in MDCK cells. Am J Physiol. 1995;268:C402–C412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases