Metabolic gene regulation in a dynamically changing environment - PubMed (original) (raw)
. 2008 Aug 28;454(7208):1119-22.
doi: 10.1038/nature07211. Epub 2008 Jul 30.
Affiliations
- PMID: 18668041
- PMCID: PMC2654342
- DOI: 10.1038/nature07211
Metabolic gene regulation in a dynamically changing environment
Matthew R Bennett et al. Nature. 2008.
Abstract
Natural selection dictates that cells constantly adapt to dynamically changing environments in a context-dependent manner. Gene-regulatory networks often mediate the cellular response to perturbation, and an understanding of cellular adaptation will require experimental approaches aimed at subjecting cells to a dynamic environment that mimics their natural habitat. Here we monitor the response of Saccharomyces cerevisiae metabolic gene regulation to periodic changes in the external carbon source by using a microfluidic platform that allows precise, dynamic control over environmental conditions. We show that the metabolic system acts as a low-pass filter that reliably responds to a slowly changing environment, while effectively ignoring fast fluctuations. The sensitive low-frequency response was significantly faster than in predictions arising from our computational modelling, and this discrepancy was resolved by the discovery that two key galactose transcripts possess half-lives that depend on the carbon source. Finally, to explore how induction characteristics affect frequency response, we compare two S. cerevisiae strains and show that they have the same frequency response despite having markedly different induction properties. This suggests that although certain characteristics of the complex networks may differ when probed in a static environment, the system has been optimized for a robust response to a dynamically changing environment.
Figures
Figure 1
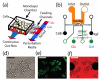
Design and implementation of the microfluidic platform developed for our study. (a) Conceptual design of the imaging chamber. The chamber is coupled to the switch output channel via multiple 1 _μ_m tall “feeding” channels. The feeding channels are fed by a controllable wave-form generator that creates sinusoidal perturbations in the glucose concentration while maintaining constant background levels of galactose. (b) An overview of the design shows the layout of the device. The device makes use of three flow networks for (1) loading cells (middle, black), (2) generating microenvironmental waveforms (bottom, green), (3) and controlling on-chip temperature (top, orange). The imaging chamber (center, gray region) is designed to be about 4 _μ_m tall in order to constrain a population of yeast cells to grow in a monolayer. (c) Representative brightfield image of cells growing in the imaging chamber. These images are used to measure the total size of the colony. Scale bar is 25 _μ_m in length, and large circles are support posts in the chamber. (d) Green fluorescence image of the same cells as in (c). These images allow us to measure the amount of Gal1p in each cell. (e) Red fluorescence image of the chamber. The glucose media also contains a red fluorescent dye, and therefore the intensity of the red fluorescence is proportional to the amount of glucose in the chamber at any given time.
Figure 2
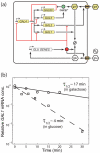
Regulation in the galactose utilization network (a) Schematic of the gene regulatory networks involved. The regulatory genes in the galactose network are activated by the Gal4p protein which binds to upstream activation sites. The GAL80 gene provides negative feedback in the system by prohibiting the inducing affects of Gal4p. Positive feedback is provided by both GAL2 and GAL3. Internalized galactose can bind to Gal3p and the resulting complex binds to Gal80p. Gal80p bound to the Gal3p-galactose complex is incapable of repressing Gal4p. Also, the transporter Gal2p increases the amount of internal galactose which stimulates the galactose network. The glucose network inhibits the transport of galactose and represses transcription of the galactose network in the presence of glucose through the action of Mig1p, which can bind to upstream regulatory sites of GAL1, GAL3 and GAL4. The glucose network also regulates the hexose transporter genes (HXT) which are responsible for transporting glucose into the cell, which then activates the glucose network. (b) Experimentally measured decay of GAL1 transcripts in galactose (circles) and glucose (squares). Also shown are the best-fit lines corresponding to half-lives of around 17 min in galactose (solid line) and 4 min in glucose (dashed line), similar to the values predicted by the numerical model. Data is normalized to the initial concentration of mRNA predicted by the best-fit lines. Similar results for GAL3 transcripts are shown in the Supplementary Information.
Figure 3
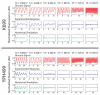
Experimental and computational results for cells expressing a GAL1-yECFP fusion gene in response to alternating glucose and galactose media for strains K699 and YPH499. The top row of each strain depicts the input glucose signal measured during each experimental run and also used to simulate the responses. The mean fluorescence of a red tracer dye, representing the glucose concentration in the media, is normalized and subtracted from 1 to represent the “induction” signal used in the experimental and computational runs above. The middle rows show normalized and detrended fluorescence trajectories for a population of cells as they respond to glucose waves of various frequencies over a galactose background. In the absence of glucose, galactose induces transcription of GAL1-yECFP causing an increase in cellular fluorescence. However, as glucose is introduced into the extracellular environment, transcription of the galactose enzymes is shut off, causing a decrease in fluorescence signal as the Gal1p-yECFP protein is degraded. Oscillation periods shown from left to right are 4.5, 3.0, 2.25, 1.5, 1.125, and 0.75 hr. For input waves with a period shorter than 1.125 hr, cells no longer respond to sinusoidal repression in a periodic fashion, demonstrating their ability to “filter” out high frequency environmental fluctuations. The bottom rows show simulation results for the same frequencies as above. The model, calibrated to experimental induction and repression data, accurately reproduces the cellular responses over a large range of frequencies.
Figure 4
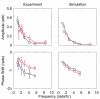
Experimental and computational comparison of two yeast strains, one of which (YPH499) is known to have a deficiency in the galactose utilization network. Amplitude (top row) and phase shift (bottom row) of the response of cells to sinusoidal repression at various frequencies are shown for both K699 (red) and YPH499 (blue) strains. For the highest frequency trial, reliable phases could not be calculated at all due to the noise, and have been omitted from the graphs. The experimental data (left column) demonstrate that the amplitude responses of the two strains are strikingly similar, especially considering their significantly different induction curves (see Supplementary Information). This phenomenon was predicted by model simulations, as slight modifications to the model parameters that affected induction and repression curves did not affect the cell population's robust response to a dynamic environment. This suggests that the complex structure of the glucose and galactose networks may confer robustness to cells even if faced with seemingly detrimental network deficiencies. The phase responses (bottom row) of the two strains did show a marked difference, with YPH499 cells exhibiting a greater phase lag than K699.
Comment in
- Systems biology: Reverse engineering the cell.
Ingolia NT, Weissman JS. Ingolia NT, et al. Nature. 2008 Aug 28;454(7208):1059-62. doi: 10.1038/4541059a. Nature. 2008. PMID: 18756243 No abstract available.
Similar articles
- Systems biology: Reverse engineering the cell.
Ingolia NT, Weissman JS. Ingolia NT, et al. Nature. 2008 Aug 28;454(7208):1059-62. doi: 10.1038/4541059a. Nature. 2008. PMID: 18756243 No abstract available. - Growth-related model of the GAL system in Saccharomyces cerevisiae predicts behaviour of several mutant strains.
Pannala VR, Hazarika SJ, Bhat PJ, Bhartiya S, Venkatesh KV. Pannala VR, et al. IET Syst Biol. 2012 Apr;6(2):44-53. doi: 10.1049/iet-syb.2010.0060. IET Syst Biol. 2012. PMID: 22519357 - Glucose- and nitrogen sensing and regulatory mechanisms in Saccharomyces cerevisiae.
Rødkaer SV, Faergeman NJ. Rødkaer SV, et al. FEMS Yeast Res. 2014 Aug;14(5):683-96. doi: 10.1111/1567-1364.12157. Epub 2014 May 8. FEMS Yeast Res. 2014. PMID: 24738657 Review. - A comparative analysis of the GAL genetic switch between not-so-distant cousins: Saccharomyces cerevisiae versus Kluyveromyces lactis.
Rubio-Texeira M. Rubio-Texeira M. FEMS Yeast Res. 2005 Dec;5(12):1115-28. doi: 10.1016/j.femsyr.2005.05.003. Epub 2005 Jul 1. FEMS Yeast Res. 2005. PMID: 16014343 Review.
Cited by
- Single-Cell Microfluidics: A Primer for Microbiologists.
Ripandelli RAA, van Oijen AM, Robinson A. Ripandelli RAA, et al. J Phys Chem B. 2024 Oct 24;128(42):10311-10328. doi: 10.1021/acs.jpcb.4c02746. Epub 2024 Oct 14. J Phys Chem B. 2024. PMID: 39400277 Free PMC article. Review. - Dynamic control of endogenous metabolism with combinatorial logic circuits.
Moser F, Espah Borujeni A, Ghodasara AN, Cameron E, Park Y, Voigt CA. Moser F, et al. Mol Syst Biol. 2018 Nov 27;14(11):e8605. doi: 10.15252/msb.20188605. Mol Syst Biol. 2018. PMID: 30482789 Free PMC article. - Microfluidic multi-analyte gradient generator.
Cao L, Zhang X, Grimley A, Lomasney AR, Roper MG. Cao L, et al. Anal Bioanal Chem. 2010 Nov;398(5):1985-91. doi: 10.1007/s00216-010-4168-8. Epub 2010 Sep 11. Anal Bioanal Chem. 2010. PMID: 20835814 Free PMC article. - Three-dimensional patterning of multiple cell populations through orthogonal genetic control of cell motility.
MacKay JL, Sood A, Kumar S. MacKay JL, et al. Soft Matter. 2014 Apr 14;10(14):2372-80. doi: 10.1039/c3sm52265b. Soft Matter. 2014. PMID: 24622945 Free PMC article. - Trade-off between responsiveness and noise suppression in biomolecular system responses to environmental cues.
Ratushny AV, Shmulevich I, Aitchison JD. Ratushny AV, et al. PLoS Comput Biol. 2011 Jun;7(6):e1002091. doi: 10.1371/journal.pcbi.1002091. Epub 2011 Jun 30. PLoS Comput Biol. 2011. PMID: 21738459 Free PMC article.
References
- Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 1961;3:318–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases