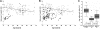TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes - PubMed (original) (raw)
TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes
Amanda J Walne et al. Blood. 2008.
Abstract
Dyskeratosis congenita (DC) is a multisystem bone marrow failure syndrome characterized by a triad of mucocutaneous abnormalities and a predisposition to cancer. The genetic basis of DC remains unknown in more than 60% of patients. Mutations have been identified in components of the telomerase complex (dyskerin, TERC, TERT, NOP10, and NHP2), and recently in one component of the shelterin complex TIN2 (gene TINF2). To establish the role of TINF2 mutations, we screened DNA from 175 uncharacterised patients with DC as well as 244 patients with other bone marrow failure disorders. Heterozygous coding mutations were found in 33 of 175 previously uncharacterized DC index patients and 3 of 244 other patients. A total of 21 of the mutations affected amino acid 282, changing arginine to histidine (n = 14) or cysteine (n = 7). A total of 32 of 33 patients with DC with TINF2 mutations have severe disease, with most developing aplastic anaemia by the age of 10 years. Telomere lengths in patients with TINF2 mutations were the shortest compared with other DC subtypes, but TERC levels were normal. In this large series, TINF2 mutations account for approximately 11% of all DC, but they do not play a significant role in patients with related disorders. This study emphasises the role of defective telomere maintenance on human disease.
Figures
Figure 1
Conservation of amino acids in a small region of TIN2 between different species. Close-up of the residues mutated in the patients with DC. Arrows indicate the sites of mutations. Changes are K280X (n = 2), R282C (n = 7), R282H (n = 14), P283A, P283H, P283S, T284A, T284Hfs8X, L287P, P289S, F290LfsX2, R291G, and Q298RfsX19. Red indicates highly conserved; blue, limited conservation; black, no conservation. Alignment obtained using MultAlin.
Figure 2
Telomere lengths in patients with DC with TINF2 mutations are the shortest compared with other DC subtypes. (A) Telomere lengths in healthy controls (○, n = 112), patients (♦, n = 16) and parents ( , n = 15). (B) Telomere length compassion between DC patients with TINF2 mutations (♦, n = 16), DKC1 mutations (
, n = 15). (B) Telomere length compassion between DC patients with TINF2 mutations (♦, n = 16), DKC1 mutations ( , n = 67) and healthy controls (○, n = 112). The line of best fit for the healthy controls is shown in panels A and B. (C) Comparison of age-adjusted delta Tel in healthy controls and patients with DKC1 and TINF2 mutations. The box represents the interquartile range which contains the 50% of values. The whiskers are lines that extend from the box to the highest and lowest values, excluding outliers and extreme outliers (○ and
, n = 67) and healthy controls (○, n = 112). The line of best fit for the healthy controls is shown in panels A and B. (C) Comparison of age-adjusted delta Tel in healthy controls and patients with DKC1 and TINF2 mutations. The box represents the interquartile range which contains the 50% of values. The whiskers are lines that extend from the box to the highest and lowest values, excluding outliers and extreme outliers (○ and  , respectively). A line across the box indicates the median.
, respectively). A line across the box indicates the median.
Figure 3
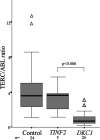
TERC/ABL levels are not reduced in patients with DC with TINF2 mutations. Comparison of TERC/ABL levels between healthy controls (n = 24), patients with DC with TINF2 mutations (n = 5), and patients with DC with DKC1 mutations (n = 26). The P value between TERC/ABL levels for TINF2 and DKC1 patients is given. The box represents the interquartile range which contains the 50% of values. The whiskers are lines that extend from the box to the highest and lowest values, excluding outliers (△). A line across the box indicates the median.
Similar articles
- TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita.
Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. Savage SA, et al. Am J Hum Genet. 2008 Feb;82(2):501-9. doi: 10.1016/j.ajhg.2007.10.004. Epub 2008 Jan 31. Am J Hum Genet. 2008. PMID: 18252230 Free PMC article. - Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood.
Sasa GS, Ribes-Zamora A, Nelson ND, Bertuch AA. Sasa GS, et al. Clin Genet. 2012 May;81(5):470-8. doi: 10.1111/j.1399-0004.2011.01658.x. Epub 2011 Apr 7. Clin Genet. 2012. PMID: 21477109 Free PMC article. - Telomere length measurement can distinguish pathogenic from non-pathogenic variants in the shelterin component, TIN2.
Vulliamy T, Beswick R, Kirwan MJ, Hossain U, Walne AJ, Dokal I. Vulliamy T, et al. Clin Genet. 2012 Jan;81(1):76-81. doi: 10.1111/j.1399-0004.2010.01605.x. Epub 2011 Jan 4. Clin Genet. 2012. PMID: 21199492 Free PMC article. - Advances in the understanding of dyskeratosis congenita.
Walne AJ, Dokal I. Walne AJ, et al. Br J Haematol. 2009 Apr;145(2):164-72. doi: 10.1111/j.1365-2141.2009.07598.x. Epub 2009 Feb 4. Br J Haematol. 2009. PMID: 19208095 Free PMC article. Review. - Dyskeratosis congenita, stem cells and telomeres.
Kirwan M, Dokal I. Kirwan M, et al. Biochim Biophys Acta. 2009 Apr;1792(4):371-9. doi: 10.1016/j.bbadis.2009.01.010. Epub 2009 Feb 7. Biochim Biophys Acta. 2009. PMID: 19419704 Free PMC article. Review.
Cited by
- When to consider inherited marrow failure syndromes in adults.
Gutierrez-Rodrigues F, Patel BA, Groarke EM. Gutierrez-Rodrigues F, et al. Hematology Am Soc Hematol Educ Program. 2023 Dec 8;2023(1):548-555. doi: 10.1182/hematology.2023000488. Hematology Am Soc Hematol Educ Program. 2023. PMID: 38066926 Free PMC article. - Differential impact of a dyskeratosis congenita mutation in TPP1 on mouse hematopoiesis and germline.
Graniel JV, Bisht K, Friedman A, White J, Perkey E, Vanderbeck A, Moroz A, Carrington LJ, Brandstadter JD, Allen F, Shami AN, Thomas P, Crayton A, Manzor M, Mychalowych A, Chase J, Hammoud SS, Keegan CE, Maillard I, Nandakumar J. Graniel JV, et al. Life Sci Alliance. 2021 Oct 13;5(1):e202101208. doi: 10.26508/lsa.202101208. Print 2022 Jan. Life Sci Alliance. 2021. PMID: 34645668 Free PMC article. - Recognizing familial myeloid leukemia in adults.
Nickels EM, Soodalter J, Churpek JE, Godley LA. Nickels EM, et al. Ther Adv Hematol. 2013 Aug;4(4):254-69. doi: 10.1177/2040620713487399. Ther Adv Hematol. 2013. PMID: 23926458 Free PMC article. - Impact of genomic damage and ageing on stem cell function.
Behrens A, van Deursen JM, Rudolph KL, Schumacher B. Behrens A, et al. Nat Cell Biol. 2014 Mar;16(3):201-7. doi: 10.1038/ncb2928. Nat Cell Biol. 2014. PMID: 24576896 Free PMC article. Review. - Dysfunctional telomeres and hematological disorders.
Fiorini E, Santoni A, Colla S. Fiorini E, et al. Differentiation. 2018 Mar-Apr;100:1-11. doi: 10.1016/j.diff.2018.01.001. Epub 2018 Jan 4. Differentiation. 2018. PMID: 29331736 Free PMC article. Review.
References
- Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. - PubMed
- Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. - PubMed
- Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases