Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance - PubMed (original) (raw)
Comparative Study
Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance
Nazish Sayed et al. Circ Res. 2008.
Abstract
Nitrates such as nitroglycerin (GTN) and nitric oxide donors such as S-nitrosothiols are clinically vasoactive through stimulation of soluble guanylyl cyclase (sGC), which produces the second messenger cGMP. Development of nitrate tolerance, after exposure to GTN for several hours, is a major drawback to a widely used cardiovascular therapy. We recently showed that exposure to nitric oxide and to S-nitrosothiols causes S-nitrosylation of sGC, which directly desensitizes sGC to stimulation by nitric oxide. We tested the hypothesis that desensitization of sGC by S-nitrosylation is a mechanism of nitrate tolerance. Our results established that vascular tolerance to nitrates can be recapitulated in vivo by S-nitrosylation through exposure to cell membrane-permeable S-nitrosothiols and that sGC is S-nitrosylated and desensitized in the tolerant, treated tissues. We next determined that (1) GTN treatment of primary aortic smooth muscle cells induces S-nitrosylation of sGC and its desensitization as a function of GTN concentration; (2) S-nitrosylation and desensitization are prevented by treatment with N-acetyl-cysteine, a precursor of glutathione, used clinically to prevent development of nitrate tolerance; and (3) S-nitrosylation and desensitization are reversed by cessation of GTN treatment. Finally, we demonstrated that in vivo development of nitrate tolerance and crosstolerance by 3-day chronic GTN treatment correlates with S-nitrosylation and desensitization of sGC in tolerant tissues. These results suggest that in vivo nitrate tolerance is mediated, in part, by desensitization of sGC through GTN-dependent S-nitrosylation.
Figures
Figure 1
S-nitrosocysteine (CSNO) treatment induces vascular tolerance that correlates with sGC S-nitrosylation and desensitization. (A) The relative luminal diameter increases by 2.0 ± 0.2 fold in response to SNAP with L-Cys treatment in the hamster cheek pouch. (B) No further vasodilation was observed in response to 10µM of SNAP after treatment with 10µM CSNO for 5min; n=4. 10µM CSNO was used as we did not observe vasodilation in the presence of 1µM CSNO and observed persistent vasodilation if 1mM CSNO was applied. (C) Western blots (WB) with anti-sGC following IP with anti-SNO show that S-nitrosylated sGC is pulled down from the CSNO-treated pouches but not from L-Cys-treated pouches or naïve controls. Inputs represent 30% of the precleared cytosols and indicate similar levels of sGC in the various samples. No sGC was pulled down with IgG indicating specificity of the anti-SNO antibodies. (D) WB with anti-sGC of biotin-avidin assay before (middle panel) and after IP with anti-sGC (right panel) indicates that sGC S-nitrosylation increased in CSNO-treated tissues. Tissues treated with 1µM CSNO did not have any detectable S-nitrosylated sGC (not shown). The sGC contains α1 and β1 subunits with a molecular weight (M.W) of 80 and 72 kDa, indicated by arrows. (D) Basal and NO-stimulated activity of the same cytosols showed that sGC from CSNO-treated tissues did not respond to NO stimulation compared to L-Cys and naïve controls. SNAP was used at 1mM. Experiments were repeated 4 times with each measurement done in duplicate and expressed in pmol/min. mg ± SE. *, P< 0.05 CSNO treated vs. naïve or L-Cys.
Figure 2
S-nitrosoglutathione (GSNO), a non-permeable S-nitrosothiol, does not induce vascular tolerance or S-nitrosylation of sGC in vivo. (A) SNAP (10µM, 5min) induces marked relaxation in the cheek pouch. (B) Similar relaxation with SNAP was observed after GSNO treatment (10µM, 5min). There was no significant difference in the arterioles luminal diameter in response to SNAP in control vs. GSNO. n=3. (C) Biotin-avidin assay followed by Western Blot with anti-sGC of 2 cheek pouches treated with GSNO and SNAP did not show S-nitrosylation. sGC was expressed in the cheek pouches (Input). Positive control of the assay indicates S-nitrosylation of WT after treatment with GSNO.
Figure 3
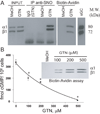
GTN treatment of primary aortic SMC induces S-nitrosylation and desensitization of endogenous sGC. (A) IP with anti-SNO (left panel) and biotin-avidin assay (right panel) indicate S-nitrosylation of sGC by GTN. Input (10% of cytosols) showed equal amount of sGC in the various cytosols. These blots are representative of 3 experiments with identical results. (B) Desensitization and S-nitrosylation of sGC is dose-dependent function of GTN. NO-stimulated cGMP production by SMC decreases with increased concentrations of GTN. Before SNAP application, the levels of cGMP were between 2.26 and 10.62 fmol/106cells for pretreatment with MeOH and 500µM GTN, respectively. These experiments were repeated 3 times with each RIA done in duplicate. SNAP: 100µM. Inset is a biotin-avidin assay showing that levels of S-nitrosylated sGC increased with increasing concentrations of GTN.
Figure 4
GTN-induced S-nitrosylation and desensitization of sGC are prevented by NAC treatment in SMC. (A) WB with anti-sGC shows that the amount of sGC pulled down with anti-SNO is greatly reduced in the NAC + GTN cytosols compared to cells not pretreated with NAC. Cells treated with MeOH or MeOH +NAC did not contain detectable S-nitrosylated sGC. GTN was used at 100µM. Inputs indicate a similar amount of sGC in the precleared cytosols of the various treatments. (B) The cytosols pretreated with NAC are more responsive to NO-stimulation compared to the ones treated with GTN only. SNAP: 100µM. The basal sGC activity was similar for the various treatments. NAC had no effect on NO-stimulated activity for samples treated with MeOH. (C) WB of biotin-avidin assay indicating that washout of GTN for 1h decreases S-nitrosylation of sGC to level close to control. Conversely, washout resulted in a drop of desensitization (to 5%) in response to 100µM SNAP. Measurements of sGC activity were done in 3 independent experiments and expressed as mean ± SE. *, P< 0.05 GTN + NAC vs. GTN
Figure 5
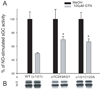
Mutations α1C243A and β1C122A significantly alter GTN-dependent desensitization. (A) COS-7 cells expressing wild-type (WT) and mutants were treated for 1h with GTN 100µM or methanol (MeOH). NO-stimulated sGC activity was measured in the cytosols with 100µM SNAP. These experiments were repeated 4 times with 3 transfections; results are expressed as the percentage of NO-stimulated activity with GTN treatment vs. vehicle. *, P<0.05 (B) WB with anti-sGC indicates that expression of sGC and mutants is not altered by GTN.
Figure 6
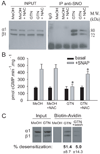
sGC is heavily S-nitrosylated and desensitized to NO and nitrates in a chronic model of nitrate tolerance. (A) 10µM GTN induces vasorelaxation in the cheek pouch of hamsters treated with propylene glycol (PG) while no relaxation is seen in the 3-day GTN-treated animals, n=3 175133/R2 23 (B) WB of biotin-avidin assays indicate high levels of S-nitrosylated sGC in the pouches (left panel) and lungs (right panel) of animals treated with GTN. (C)WB with anti-sGC of IP with anti-SNO confirmed that GTN-tolerant tissues have higher levels of S-nitrosylated sGC than PG-treated tissues. Correspondingly, less un-nitrosylated sGC is present in the unbound fraction in tolerant tissues. (D) cGMP-production in response to SNAP or GTN is significantly impaired in the lungs of GTN-treated animals. Similar results were obtained with the pouches (inset). The levels of cGMP were too low with 10µM GTN over basal levels to obtain significant stimulation-fold values in the pouches. n=4; *, P<0.05 sham vs. GTN-treated. (E) 10µM SNAP induces vasorelaxation in the cheek pouch of hamsters treated with PG while significantly less relaxation was seen in the GTN-treated animals, n=5; P<0.05 Area Under the Curve PG vs. GTN.
Comment in
- Nitroglycerin-mediated S-nitrosylation of proteins: a field comes full cycle.
Stamler JS. Stamler JS. Circ Res. 2008 Sep 12;103(6):557-9. doi: 10.1161/CIRCRESAHA.108.184341. Circ Res. 2008. PMID: 18796638 Free PMC article. No abstract available.
Similar articles
- Effect of soluble guanylyl cyclase activator and stimulator therapy on nitroglycerin-induced nitrate tolerance in rats.
Jabs A, Oelze M, Mikhed Y, Stamm P, Kröller-Schön S, Welschof P, Jansen T, Hausding M, Kopp M, Steven S, Schulz E, Stasch JP, Münzel T, Daiber A. Jabs A, et al. Vascul Pharmacol. 2015 Aug;71:181-91. doi: 10.1016/j.vph.2015.03.007. Epub 2015 Apr 11. Vascul Pharmacol. 2015. PMID: 25869522 - Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation.
Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. Sayed N, et al. Proc Natl Acad Sci U S A. 2007 Jul 24;104(30):12312-7. doi: 10.1073/pnas.0703944104. Epub 2007 Jul 16. Proc Natl Acad Sci U S A. 2007. PMID: 17636120 Free PMC article. - In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics.
Artz JD, Toader V, Zavorin SI, Bennett BM, Thatcher GR. Artz JD, et al. Biochemistry. 2001 Aug 7;40(31):9256-64. doi: 10.1021/bi002885x. Biochemistry. 2001. PMID: 11478893 - The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles.
Mayer B, Beretta M. Mayer B, et al. Br J Pharmacol. 2008 Sep;155(2):170-84. doi: 10.1038/bjp.2008.263. Epub 2008 Jun 23. Br J Pharmacol. 2008. PMID: 18574453 Free PMC article. Review. - Heme-dependent and independent soluble guanylate cyclase activators and vasodilation.
Priviero FB, Webb RC. Priviero FB, et al. J Cardiovasc Pharmacol. 2010 Sep;56(3):229-33. doi: 10.1097/FJC.0b013e3181eb4e75. J Cardiovasc Pharmacol. 2010. PMID: 20571429 Free PMC article. Review.
Cited by
- Glutathione and thioredoxin type 1 cooperatively denitrosate HepG2 cells-derived cytosolic S-nitrosoproteins.
Stoyanovsky DA, Scott MJ, Billiar TR. Stoyanovsky DA, et al. Org Biomol Chem. 2013 Jul 21;11(27):4433-7. doi: 10.1039/c3ob40809d. Org Biomol Chem. 2013. PMID: 23743503 Free PMC article. - Glyceryl Trinitrate: History, Mystery, and Alcohol Intolerance.
Pearson R, Butler A. Pearson R, et al. Molecules. 2021 Oct 30;26(21):6581. doi: 10.3390/molecules26216581. Molecules. 2021. PMID: 34770988 Free PMC article. Review. - Dynamic ligand exchange in soluble guanylyl cyclase (sGC): implications for sGC regulation and desensitization.
Tsai AL, Berka V, Sharina I, Martin E. Tsai AL, et al. J Biol Chem. 2011 Dec 16;286(50):43182-92. doi: 10.1074/jbc.M111.290304. Epub 2011 Oct 18. J Biol Chem. 2011. PMID: 22009742 Free PMC article. - Nitroglycerin-mediated S-nitrosylation of proteins: a field comes full cycle.
Stamler JS. Stamler JS. Circ Res. 2008 Sep 12;103(6):557-9. doi: 10.1161/CIRCRESAHA.108.184341. Circ Res. 2008. PMID: 18796638 Free PMC article. No abstract available. - The nitric oxide pathway and possible therapeutic options in pre-eclampsia.
Johal T, Lees CC, Everett TR, Wilkinson IB. Johal T, et al. Br J Clin Pharmacol. 2014 Aug;78(2):244-57. doi: 10.1111/bcp.12301. Br J Clin Pharmacol. 2014. PMID: 24313856 Free PMC article. Review.
References
- Hashimoto S, Kobayashi A. Clinical pharmacokinetics and pharmacodynamics of glyceryl trinitrate and its metabolites. Clin Pharmacokinet. 2003;42:205–221. - PubMed
- Fung HL. Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu Rev Pharmacol Toxicol. 2004;44:67–85. - PubMed
- Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–628. - PubMed
- Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM067640/GM/NIGMS NIH HHS/United States
- HL089771/HL/NHLBI NIH HHS/United States
- R01 GM067640-05/GM/NIGMS NIH HHS/United States
- GM067640/GM/NIGMS NIH HHS/United States
- R21 HL089771/HL/NHLBI NIH HHS/United States
- R01 HL070634/HL/NHLBI NIH HHS/United States
- HL070634/HL/NHLBI NIH HHS/United States
- R21 HL089771-01A1/HL/NHLBI NIH HHS/United States
- R01 HL070634-04/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources