Cell-based therapy in ischemic stroke - PubMed (original) (raw)
Review
Cell-based therapy in ischemic stroke
David C Hess et al. Expert Rev Neurother. 2008 Aug.
Abstract
Cell-based therapy for stroke represents a third wave of therapeutics for stroke and one focused on restorative processes with a longer time window of opportunity than neuroprotective therapies. An early time window, within the first week after stroke, is an opportunity for intravenously delivered bone marrow and perinatally derived cells that can home to areas of tissue injury and target brain remodeling. Allogeneic cells will likely be the most scalable and commercially viable product. Later time windows, months after stroke, may be opportunities for intracerebral transplantation of neuronally differentiated cell types. An integrated approach of cell-based therapy with early-phase clinical trials and continued preclinical work with focus on mechanisms of action is needed.
Figures
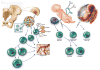
Figure 1
The bone marrow is a rich source of stem and progenitor cells. Hematopoietic stem cells (HSC) give rise to CD133+, CD34+, and cells that are both CD34+ and CD133+. Marrow stromal cells (MSC) differentiate into multiple cells types. Rare cell types such as the multipotent adult progenitor cell and MIAMI cells can be expanded in culture and differentiate into cells of all three germ layers. The umbilical cord, the amniotic fluid and the placenta are sources of cell types with regenerative potential.
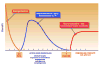
Figure 2
Depiction of the three “time windows of opportunity” available for stroke. The neuroprotective window is short, lasting hours. The subacute window, is an area of opportunity for intravascularly administered cells by an intra-arterial or intravenous route. Neurotrophic factors will also play an important role in this window. “Chronic stroke” will likely require stereotactic surgical approaches with direct intracerebral delivery of cells differentiated down a neuronal lineage.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Falls prevention interventions for community-dwelling older adults: systematic review and meta-analysis of benefits, harms, and patient values and preferences.
Pillay J, Gaudet LA, Saba S, Vandermeer B, Ashiq AR, Wingert A, Hartling L. Pillay J, et al. Syst Rev. 2024 Nov 26;13(1):289. doi: 10.1186/s13643-024-02681-3. Syst Rev. 2024. PMID: 39593159 Free PMC article. - Evaluating Different Types of Cancer Survivorship Care [Internet].
Mead KH, Raskin S, Arem H, Landry M, Seyoum S, Cleary S, Pratt-Chapman M. Mead KH, et al. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jul. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jul. PMID: 39602557 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Continuing education meetings and workshops: effects on professional practice and healthcare outcomes.
Forsetlund L, O'Brien MA, Forsén L, Reinar LM, Okwen MP, Horsley T, Rose CJ. Forsetlund L, et al. Cochrane Database Syst Rev. 2021 Sep 15;9(9):CD003030. doi: 10.1002/14651858.CD003030.pub3. Cochrane Database Syst Rev. 2021. PMID: 34523128 Free PMC article. Review.
Cited by
- Toward personalized cell therapies: autologous menstrual blood cells for stroke.
Rodrigues MC, Glover LE, Weinbren N, Rizzi JA, Ishikawa H, Shinozuka K, Tajiri N, Kaneko Y, Sanberg PR, Allickson JG, Kuzmin-Nichols N, Garbuzova-Davis S, Voltarelli JC, Cruz E, Borlongan CV. Rodrigues MC, et al. J Biomed Biotechnol. 2011;2011:194720. doi: 10.1155/2011/194720. Epub 2011 Nov 20. J Biomed Biotechnol. 2011. PMID: 22162629 Free PMC article. Review. - Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats.
Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Xin H, et al. J Cereb Blood Flow Metab. 2013 Nov;33(11):1711-5. doi: 10.1038/jcbfm.2013.152. Epub 2013 Aug 21. J Cereb Blood Flow Metab. 2013. PMID: 23963371 Free PMC article. - Stem cell therapy as a promising approach for ischemic stroke treatment.
Yaqubi S, Karimian M. Yaqubi S, et al. Curr Res Pharmacol Drug Discov. 2024 May 16;6:100183. doi: 10.1016/j.crphar.2024.100183. eCollection 2024. Curr Res Pharmacol Drug Discov. 2024. PMID: 38831867 Free PMC article. Review. - The circular RNA Rap1b promotes Hoxa5 transcription by recruiting Kat7 and leading to increased Fam3a expression, which inhibits neuronal apoptosis in acute ischemic stroke.
Zhang FF, Zhang L, Zhao L, Lu Y, Dong X, Liu YQ, Li Y, Guo S, Zheng SY, Xiao Y, Jiang YZ. Zhang FF, et al. Neural Regen Res. 2023 Oct;18(10):2237-2245. doi: 10.4103/1673-5374.369115. Neural Regen Res. 2023. PMID: 37056143 Free PMC article. - Adult stem cell transplantation: is gender a factor in stemness?
Tajiri N, Duncan K, Borlongan MC, Pabon M, Acosta S, de la Pena I, Hernadez-Ontiveros D, Lozano D, Aguirre D, Reyes S, Sanberg PR, Eve DJ, Borlongan CV, Kaneko Y. Tajiri N, et al. Int J Mol Sci. 2014 Aug 28;15(9):15225-43. doi: 10.3390/ijms150915225. Int J Mol Sci. 2014. PMID: 25170809 Free PMC article. Review.
References
- Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39. - PubMed
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008 - PubMed
- Nadal-Ginard B, Fuster V. Myocardial cell therapy at the crossroads. Nat Clin Pract Cardiovasc Med. 2007;4(1):1. - PubMed
- * An editorial calling for a moratorium on cell-based therapy clinical trials.
- Cardiac cell therapy: bench or bedside? Nat Clin Pract Cardiovasc Med. 2007;4(8):403. - PubMed
- * A counter argument to the editorial calling for a moratorium on cell-based therapy in cardiovascular diseases.
- Bath PM, Sprigg N. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev. 2006;3:CD005207. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS055728/NS/NINDS NIH HHS/United States
- R42 NS055606-02A1/NS/NINDS NIH HHS/United States
- R42 NS055606/NS/NINDS NIH HHS/United States
- U01 NS055914/NS/NINDS NIH HHS/United States
- 2R42 NS055606/NS/NINDS NIH HHS/United States
- 1U01 NS 055914/NS/NINDS NIH HHS/United States
- U01 NS055914-02/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical