Integrative analysis of RUNX1 downstream pathways and target genes - PubMed (original) (raw)
Comparative Study
doi: 10.1186/1471-2164-9-363.
Ken M Simpson, Robert Escher, Karine Buchet-Poyau, Tim Beissbarth, Catherine Carmichael, Matthew E Ritchie, Frédéric Schütz, Ping Cannon, Marjorie Liu, Xiaofeng Shen, Yoshiaki Ito, Wendy H Raskind, Marshall S Horwitz, Motomi Osato, David R Turner, Terence P Speed, Maria Kavallaris, Gordon K Smyth, Hamish S Scott
Affiliations
- PMID: 18671852
- PMCID: PMC2529319
- DOI: 10.1186/1471-2164-9-363
Comparative Study
Integrative analysis of RUNX1 downstream pathways and target genes
Joëlle Michaud et al. BMC Genomics. 2008.
Abstract
Background: The RUNX1 transcription factor gene is frequently mutated in sporadic myeloid and lymphoid leukemia through translocation, point mutation or amplification. It is also responsible for a familial platelet disorder with predisposition to acute myeloid leukemia (FPD-AML). The disruption of the largely unknown biological pathways controlled by RUNX1 is likely to be responsible for the development of leukemia. We have used multiple microarray platforms and bioinformatic techniques to help identify these biological pathways to aid in the understanding of why RUNX1 mutations lead to leukemia.
Results: Here we report genes regulated either directly or indirectly by RUNX1 based on the study of gene expression profiles generated from 3 different human and mouse platforms. The platforms used were global gene expression profiling of: 1) cell lines with RUNX1 mutations from FPD-AML patients, 2) over-expression of RUNX1 and CBFbeta, and 3) Runx1 knockout mouse embryos using either cDNA or Affymetrix microarrays. We observe that our datasets (lists of differentially expressed genes) significantly correlate with published microarray data from sporadic AML patients with mutations in either RUNX1 or its cofactor, CBFbeta. A number of biological processes were identified among the differentially expressed genes and functional assays suggest that heterozygous RUNX1 point mutations in patients with FPD-AML impair cell proliferation, microtubule dynamics and possibly genetic stability. In addition, analysis of the regulatory regions of the differentially expressed genes has for the first time systematically identified numerous potential novel RUNX1 target genes.
Conclusion: This work is the first large-scale study attempting to identify the genetic networks regulated by RUNX1, a master regulator in the development of the hematopoietic system and leukemia. The biological pathways and target genes controlled by RUNX1 will have considerable importance in disease progression in both familial and sporadic leukemia as well as therapeutic implications.
Figures
Figure 1
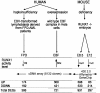
Gene expression profiles and overlaps. The three platforms used in this study are indicated. The number of up-, down- or all differentially expressed genes (DEGs) are indicated below each platform.
Figure 2
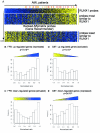
Correlation with clinical AML data. A. Published microarray data on 285 AML patients [23] were ordered using Gene Recommender according to the expression pattern of the 11 probe sets for RUNX1. The patients with t(8;21) are marked in orange and those with inv(16) in red. Probes co-regulated with RUNX1 are highly ranked (yellow bar), whereas probes showing an expression pattern the least similar to RUNX1 are ranked lowest (blue bar). B-C. Random permutations were performed to compare the rank of the genes differentially expressed in FPD platform and random set of genes. The histograms show the percentage of up- or down-regulated genes in FPD relative to their rank with "0" being the probes co-regulated with RUNX1 (yellow) and "1" being the probes the least similar to RUNX1 (blue). The trends observed in the histograms are represented as triangles or rectangle. D-E. Similar histograms showing percentage of up- or down-regulated genes in CBF relative to their rank.
Figure 3
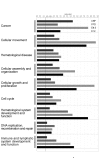
Processes identified by Ingenuity Pathways Analysis. Evidence that each dataset is involved in the given function as determined by the use of Ingenuity Pathways Analysis (Ingenuity Systems,
). The threshold for the significance is indicated by a vertical bar and represents a p-value of 0.05.
Figure 4
MR-GSE test. Representation of the p-values (corrected for multiple testing) resulting from the MR-GSE test for each dataset and 10 gene sets specified in Additional File 1 (Table S4). In brief they are gene sets Mekagaryocyte differentiation, Identification of genes involved in the differentiation of megakaryocytes. DEGs between stem cells and differentiated megakaryocytes; Platelets, Transcription profiling of human blood platelet; ; Normal megakaryocytes, Genes highly expressed in megakaryocytes; ET megakaryocytes, Genes highly expressed in essential thrombocytopenia megakaryocytes; Cytokinesis proteome, Identification of proteins present in the midbody during cytokinesis; Spindle checkpoint, Review ; DNA repair, Review; Lymphoblast irradiation; high dose, Effect of ionising radiation on lymphoblasts; Lymphoblast irradiation; low dose, Effect of ionising radiation on lymphoblasts; Genes DE in cancer, Meta-analysis of cancer microarray data to identify genes consistently DE in tumours. This represents whether the genes present in the published gene sets are also differentially expressed in our expression profiles. For example, the genes expressed in normal or diseased megakaryocytes (lines 3 and 4) are significantly represented in the differentially expressed genes identified in the FPD and CBF approaches.
Figure 5
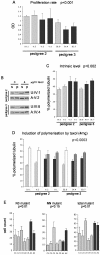
Functional assays on FPD-AML cell lines. A. The results of a BrdU proliferation assay are indicated for each cell line. Dark bars indicate affected individuals. The standard errors of two independent replicates are shown. A two-way ANOVA resulted in a significant p-value (p < 0.001) between affected and unaffected individuals. B. Examples of the tubulin polymerization assay for an affected and an unaffected individuals in each family. s:soluble tubulin; p:polymerized tubulin. C. The percentage of polymerized tubulin is shown for each cell line. Dark bars indicate affected individuals. The standard errors of three independent replicates are indicated. A two-way ANOVA resulted in a significant p-value (p < 0.002) between affected and unaffected individuals. D. Percentage of polymerized tubulin in the same cell lines before (darker left bars) and after (second bars) induction of polymerization by Taxol. A significant smaller induction is observed in affected individuals (dark bars) as demonstrated by an ANOVA (p < 0.0003). E. Glycophorin A assay. The numbers of N0 (loss of the M allele), NN (mutation changing M to N allele) or total mutant (both N0 and NN) cells are indicated for each individual. The standard errors of three to five technical replicates are indicated. Dark bars represent affected individuals (A1-A2). The control C5 is the unaffected sister of patient A1. ANOVAs were performed for each kind of mutation and the p-values are indicated.
Figure 6
A. Overlaps between the datasets and percentage of genes with a RUNX1 binding site in their regulatory regions. The overlaps between the different platforms are represented with arrows. * indicates that the genes differentially expressed in at least one of the mouse datasets are considered for the following overlap. The number of differentially expressed genes (DEGs) containing a conserved RUNX1 binding site (with CBS) in their regulatory regions, as determined by the oPOSSUM program [27], over the number of analyzed genes is indicated for each dataset and overlap. The corresponding percentage is indicated in brackets. B. Luciferase assay for 5 RUNX1 binding sites corresponding to 3 differentially expressed genes. The transactivation activity of RUNX1 over these sites was measured as the fold change of the luciferase activity in the presence of the CBF complex compared to the endogenous activity of each construct. The standard errors of three independent replicates are shown. CASP3 was shown as a negative control as no binding site was found for this gene. The difference in expression for the three genes in each dataset is indicated in the table. 0 means no difference in expression, ↓ stands for down-regulated and ↑ stands for up-regulated.
Figure 7
Part of the networks downsteam of RUNX1. Additional data from the literature and our studies were used to update the standard Ingenuity Pathway System (Ingenuity® Systems,
) network analyses. Genes up-regulated (red) or down-regulated (green) in either FPD or CBF are indicated. Selected chosen functions with significant network nodes are shown including all the genes involved in cytoskeleton organization. Grey arrows represent transcriptional regulation, grey lines represent direct interaction, dotted lines represent indirect link. Each kind of molecule is represented by a different symbol (see ).
Similar articles
- In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis.
Michaud J, Wu F, Osato M, Cottles GM, Yanagida M, Asou N, Shigesada K, Ito Y, Benson KF, Raskind WH, Rossier C, Antonarakis SE, Israels S, McNicol A, Weiss H, Horwitz M, Scott HS. Michaud J, et al. Blood. 2002 Feb 15;99(4):1364-72. doi: 10.1182/blood.v99.4.1364. Blood. 2002. PMID: 11830488 - Identification of Key Genes and Pathways Associated with RUNX1 Mutations in Acute Myeloid Leukemia Using Bioinformatics Analysis.
Zhu F, Huang R, Li J, Liao X, Huang Y, Lai Y. Zhu F, et al. Med Sci Monit. 2018 Oct 5;24:7100-7108. doi: 10.12659/MSM.910916. Med Sci Monit. 2018. PMID: 30289875 Free PMC article. - RUNX1 and CBFβ Mutations and Activities of Their Wild-Type Alleles in AML.
Hyde RK, Liu P, Friedman AD. Hyde RK, et al. Adv Exp Med Biol. 2017;962:265-282. doi: 10.1007/978-981-10-3233-2_17. Adv Exp Med Biol. 2017. PMID: 28299663 Review. - Downregulation of RUNX1/CBFβ by MLL fusion proteins enhances hematopoietic stem cell self-renewal.
Zhao X, Chen A, Yan X, Zhang Y, He F, Hayashi Y, Dong Y, Rao Y, Li B, Conway RM, Maiques-Diaz A, Elf SE, Huang N, Zuber J, Xiao Z, Tse W, Tenen DG, Wang Q, Chen W, Mulloy JC, Nimer SD, Huang G. Zhao X, et al. Blood. 2014 Mar 13;123(11):1729-38. doi: 10.1182/blood-2013-03-489575. Epub 2014 Jan 21. Blood. 2014. PMID: 24449215 Free PMC article. - Myeloid neoplasms with germ line RUNX1 mutation.
Hayashi Y, Harada Y, Huang G, Harada H. Hayashi Y, et al. Int J Hematol. 2017 Aug;106(2):183-188. doi: 10.1007/s12185-017-2258-5. Epub 2017 May 22. Int J Hematol. 2017. PMID: 28534116 Review.
Cited by
- Glutamine addiction in tumor cell: oncogene regulation and clinical treatment.
Li X, Peng X, Li Y, Wei S, He G, Liu J, Li X, Yang S, Li D, Lin W, Fang J, Yang L, Li H. Li X, et al. Cell Commun Signal. 2024 Jan 3;22(1):12. doi: 10.1186/s12964-023-01449-x. Cell Commun Signal. 2024. PMID: 38172980 Free PMC article. Review. - Pathway analysis approaches for rare and common variants: insights from Genetic Analysis Workshop 18.
Aslibekyan S, Almeida M, Tintle N. Aslibekyan S, et al. Genet Epidemiol. 2014 Sep;38 Suppl 1(0 1):S86-91. doi: 10.1002/gepi.21831. Genet Epidemiol. 2014. PMID: 25112195 Free PMC article. - ERK Phosphorylation Regulates the Aml1/Runx1 Splice Variants and the TRP Channels Expression during the Differentiation of Glioma Stem Cell Lines.
Santoni G, Nabissi M, Amantini C, Santoni M, Ricci-Vitiani L, Pallini R, Maggi F, Morelli MB. Santoni G, et al. Cells. 2021 Aug 10;10(8):2052. doi: 10.3390/cells10082052. Cells. 2021. PMID: 34440820 Free PMC article. - Neonatal DNA methylation profile in human twins is specified by a complex interplay between intrauterine environmental and genetic factors, subject to tissue-specific influence.
Gordon L, Joo JE, Powell JE, Ollikainen M, Novakovic B, Li X, Andronikos R, Cruickshank MN, Conneely KN, Smith AK, Alisch RS, Morley R, Visscher PM, Craig JM, Saffery R. Gordon L, et al. Genome Res. 2012 Aug;22(8):1395-406. doi: 10.1101/gr.136598.111. Epub 2012 Jul 16. Genome Res. 2012. PMID: 22800725 Free PMC article. - Analysis of myocardial cellular gene expression during pressure overload reveals matrix based functional intercellular communication.
Froese N, Cordero J, Abouissa A, Trogisch FA, Grein S, Szaroszyk M, Wang Y, Gigina A, Korf-Klingebiel M, Bosnjak B, Davenport CF, Wiehlmann L, Geffers R, Riechert E, Jürgensen L, Boileau E, Lin Y, Dieterich C, Förster R, Bauersachs J, Ola R, Dobreva G, Völkers M, Heineke J. Froese N, et al. iScience. 2022 Feb 23;25(3):103965. doi: 10.1016/j.isci.2022.103965. eCollection 2022 Mar 18. iScience. 2022. PMID: 35281736 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases