Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice - PubMed (original) (raw)
doi: 10.1016/j.ajhg.2008.07.008.
Sarah Emery, Régis Nouvian, Tiphaine Bersot, Bénédicte Amilhon, Jana M Van Rybroek, Guy Rebillard, Marc Lenoir, Michel Eybalin, Benjamin Delprat, Theru A Sivakumaran, Bruno Giros, Salah El Mestikawy, Tobias Moser, Richard J H Smith, Marci M Lesperance, Jean-Luc Puel
Affiliations
- PMID: 18674745
- PMCID: PMC2495073
- DOI: 10.1016/j.ajhg.2008.07.008
Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice
Jérôme Ruel et al. Am J Hum Genet. 2008 Aug.
Abstract
Autosomal-dominant sensorineural hearing loss is genetically heterogeneous, with a phenotype closely resembling presbycusis, the most common sensory defect associated with aging in humans. We have identified SLC17A8, which encodes the vesicular glutamate transporter-3 (VGLUT3), as the gene responsible for DFNA25, an autosomal-dominant form of progressive, high-frequency nonsyndromic deafness. In two unrelated families, a heterozygous missense mutation, c.632C-->T (p.A211V), was found to segregate with DFNA25 deafness and was not present in 267 controls. Linkage-disequilibrium analysis suggested that the families have a distant common ancestor. The A211 residue is conserved in VGLUT3 across species and in all human VGLUT subtypes (VGLUT1-3), suggesting an important functional role. In the cochlea, VGLUT3 accumulates glutamate in the synaptic vesicles of the sensory inner hair cells (IHCs) before releasing it onto receptors of auditory-nerve terminals. Null mice with a targeted deletion of Slc17a8 exon 2 lacked auditory-nerve responses to acoustic stimuli, although auditory brainstem responses could be elicited by electrical stimuli, and robust otoacoustic emissions were recorded. Ca(2+)-triggered synaptic-vesicle turnover was normal in IHCs of Slc17a8 null mice when probed by membrane capacitance measurements at 2 weeks of age. Later, the number of afferent synapses, spiral ganglion neurons, and lateral efferent endings below sensory IHCs declined. Ribbon synapses remaining by 3 months of age had a normal ultrastructural appearance. We conclude that deafness in Slc17a8-deficient mice is due to a specific defect of vesicular glutamate uptake and release and that VGLUT3 is essential for auditory coding at the IHC synapse.
Figures
Figure 1
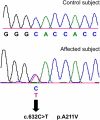
c.632C→T Mutation in SLC17A8 Sequencing chromatogram from normal-hearing control (top) shows the wild-type nucleotide C at position 632. The wild-type codon is GCA and codes for alanine. Sequencing chromatogram from affected individual from family 1 (bottom) shows heterozygous c.632C→T mutation (indicated by an arrow), leading to p.A211V amino acid change. The codon containing the mutation is GTA and the altered amino acid is valine.
Figure 2
Conservation of Amino Acids of VGLUT3 Encoded by Exons 5 and 6 Amino acid sequence representing exons 5 and 6 of select proteins from the VGLUT protein family in H. sapiens, R. norvegicus, and M. musculus, and homologous proteins in C. elegans and D. melanogaster were aligned in ClustalX2. Asterisks show identical residues between the aligned sequences. An arrow indicates residue mutated in DFNA25 deafness.
Figure 3
Slc17a8 Deletion Does Not Induce Major Change at IHC-Afferent Synapses Electron (A and C–J) and light microscopy (B) of the organ of Corti in the basal turn of _Slc17a8_−/− (A–F and H–J) and wild-type (+/+, [G]) mice are shown. (A) Surface of the organ of Corti observed with SEM. The stereociliary bundles are well organized in both the three rows of OHCs and the single row of IHCs. However, note the missing stereociliary bundle (asterisk) in the IHC row. “p” stands for pillar cells. (B) Gross morphology of the organ of Corti seen in transverse section. The organ of Corti shows a normal appearance with well opened tunnel of Corti (tC), one IHC, and three OHCs. Note the presence of medial efferent fibers (indicated by an arrowhead) crossing the tunnel of Corti. The following abbreviations are used: D, Deiters cells; tm, tectorial membrane; and bm, basilar membrane. (C)–(M) show TEM. (C) A normal appearing OHC showing erected stereocilia (indicated by an arrow), clear cytoplasm, a basal nucleus with peripherally located chromatin, typical distribution of mitochondria (indicated by arrowheads) along the lateral wall of the cell and below the nucleus. Note the efferent nerve ending (e) onto the basal pole of the OHC. (D) Normal appearing IHC with erected stereocilia (indicated by an arrow), a clear cytoplasm, and a nucleus centrally positioned with peripheral chromatin. Note the nerve fibers underneath the IHC and in contact with its basal pole (indicated by arrowheads). The following abbreviations are used: pc, phalangeal cell; bc, border cell; and ip, inner pillar cell. (E) Typical innervation pattern at the basal pole of an OHC showing a large efferent ending and a smaller afferent bouton (a). (F) Basal pole of an IHC contacted by several afferent dendrites (a). Note the presence of a vesiculated efferent fiber (e) synapsing (indicated by arrowheads) with an afferent dendrite. “sc” stands for supporting cell. (G–N) Structural variability of synaptic ribbons in IHCs from wild-type (G–J) and _Slc17a8_-deleted (K–N) mice. (G), (K), and (J) show normal synaptic ribbons. In (G) and (K), note the postsynaptic density (indicated by arrowheads) on the afferent bouton. (H)–(N) show atypical synaptic organelles including very long (H and M) and ectopic (I and N) ribbons and accumulation of synaptic vesicles without central electron-dense body (J). (O) Proportion of normal versus atypical ribbons in wild-type (n = 3 cochleas) and _Slc17a8_−/− (n = 6 cochleas) mice. Quantification was done from 18 and 30 IHCs, respectively. Percents were calculated from 19 synaptic bodies in the wild-type and 23 in the _Slc17a8_−/− mice. (P) The left panel shows quantitative analysis of the outer diameter of vesicles surrounding normal synaptic ribbons seen in IHCs from three wild-type mice (+/+) and six _Slc17a8_-deleted mice (−/−). A total of 16 synaptic ribbons were investigated in the wild-type mice against 19 synaptic ribbons in the _Slc17a8_−/− mice. A total number of 140 and 129 synaptic vesicles were measured in the wild-type and the deleted mice, respectively. The right panel shows quantification of vesicles per 100 nm of central dense body in normal synaptic ribbons from wild-type mice (n = 7 ribbons) and _Slc17a8_-deleted mice (n = 7 ribbons). No significant difference was found. Scale bars in (A) and (B) represent 10 μm, those in (C) and (D) represent 5 μm, those in (E) and (F) represent 1 μm, and those in (G)–(N) represent 100 nm.
Figure 4
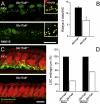
Slc17a8 Deletion Reduced the Number of IHC Synapses and Lateral Efferent Endings (A) Confocal microscopy of RIBEYE immunoreactivity through basal turn inner hair cells of Slc17a8+/+ and −/− mice. The hair cell nuclei display the immunoreactivity to the CtBP2 transcription factor. The RIBEYE-immunoreactive dots are seen at the hair cell (IHC) bases in the _Slc17a8_−/− mouse, although they are less densely distributed than in the wild-type. The inserts show that in both mice, RIBEYE-immunoreactive dots face GluR2 immunoreactive spots. The scale bar represents 15 μm. (B) RIBEYE dots count in +/+ and −/− cochleas (n = 3 and 4 respectively) shows a strong (43.90%) and significant (p = 9.17 × 10−8) decrease of the number of immunoreactive dots at the IHC bases of _SLC17A8_−/− mice. (C) Confocal microscopy through the basal turn of Slc17a8+/+ and −/− mice. IHCs are labeled with parvalbumin (red), and lateral efferent endings are labeled with synaptophysin (green). Note the strong reduction in the number of immunoreactive endings in the _Slc17a8_−/− mouse. The scale bar represents 10 μm. (D) Quantitative analysis of the number of synaptophysin-immunoreactive endings beneath IHCs from the basal and medial cochlear turns of wild-type (n = 4 cochleas) and _Slc17a8_−/− (n = 4 cochleas) mice. The reduction in the number of synaptophysin-immunoreactive endings is more pronounced in the basal turn (69.51% reduction) than in the medial turn (43.26%).
Figure 5
Slc17a8 Deletion Leads to a Profound Deafness, but Does Not Affect the Functioning of Cochlear Mechanotransduction (A) Typical examples of averaged cochlear action potentials (CAPs) recorded from an electrode apposed onto the round-window membrane for wild-type and _Slc17a8_−/− mice. The potentials were recorded in response to 12 kHz tone bursts presented from 0 to 100 dB SPL. Slc17a8+/+ (n = 12) and Slc17a8+/− heterozygous mice (n = 8, not shown) showed the classical sound-evoked N1-P1 CAP waveform in responses to all sound stimulation. In contrast, the _Slc17a8_−/− (n = 12) littermates showed no visible CAP response, even for high stimulus intensities. Note that the summating potential (SP), which reflect the functional state of the inner hair cells, remains intact in _Slc17a8_−/− mice. (B) CAP audiograms for _Slc17a8_−/−, Slc17a8+/+ and Slc17a8+/− mice were obtained by plotting CAP thresholds as a function of the stimulation frequency (2–64 kHz tone-bursts). Compared to Slc17a8+/+ (black circles) and Slc17a8+/− (open squares) mice, _Slc17a8_−/− mice (open circles) show no sound-evoked response at any stimulating frequency and intensity. (C–E) Input-output functions of the CAP, SP, and cochlear microphonic (CM) evoked by a 12 kHz tone-bursts from 0 to 100 dB SPL in _Slc17a8_−/−, Slc17a8+/+ and Slc17a8+/− mice. As shown in (C), _Slc17a8_−/− mice displayed a complete abolition of CAP. The inset in (C) shows a typical trace of averaged cochlear potentials evoked by 12 kHz tone bursts presented at 80 dB sound pressure level (SPL). By contrast, normal SP (D) and CM (E) potentials were recorded in all animals tested. (F) Recordings of distortion product otoacoustic emissions (2f1-f2) reflect the cochlear nonlinearity as a result of outer hair cell function. No significant change was measured between _Slc17a8_−/−, Slc17a8+/+, and Slc17a8+/− mice. The thick gray line indicates the background noise level of the recording system in the absence of sound. Values are mean ± SEM, and n indicates the number of animal tested.
Figure 6
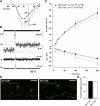
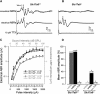
Ca2+-Triggered IHC Exocytosis Is Not Impaired by the Lack of Vglut3 (A) Ca2+ current steady-state I/V relationships of Slc17a8+/+ (black) and Slc17a8−/− (gray) IHCs from P12-18 mice. Steady-state amplitude was measured as the average over the last 5 ms of the 10 ms test pulse. Statistical significant difference is indicated by asterisks (p < 0.05, unpaired t test). (B) Ca2+ current (_I_Ca2+), membrane capacitance (_C_m) low-pass filtered at 100 Hz, series conductance (_G_s), and membrane conductance (_G_m) traces (from top to bottom) of representative Slc17a8+/+ (black, P12) and Slc17a8−/− (gray, P13) IHCs elicited by 20 ms depolarization to the peak Ca2+ current potential. (C) Kinetics of exocytosis (ΔCm, top) and corresponding Ca2+ current integrals (QCa2+, bottom) of Slc17a8+/+ (black) and Slc17a8−/− (gray) IHCs. ΔCm were obtained by multiple depolarizations of different durations to peak calcium current potential in each IHC and represent grand averages calculated from the means of the individual IHC. For Slc17a8+/+ and Slc17a8−/−, respectively, _R_s = 19.6 ± 0.7 MΩ and 18.7 ± 0.9 MΩ; resting membrane capacitance = 8.9 ± 0.2 pF and 8.9 ± 1.4 pF, and resting current at holding potential (−87 mV) = −17.7 ± 1.1 pA and −17.9 ± 1.1 pA. Statistically significant difference is indicated by asterisks (p < 0.05, unpaired t test). (D) Representative projections of confocal sections from P14 Slc17a8+/+ and P17 Slc17a8−/− apical organs of Corti stained for GluR2/3 (red) and RIBEYE/CtBP2 (green). “n” stands for nucleus; scale bars represent 5 μm. (E) Quantitative analysis of synapse-anchored ribbons per IHC identified as small RIBEYE-positive spots underneath the IHC nuclei juxtaposing GluR2/3 immunofluorescence spots from P13-P17 mice.
Figure 7
Slc17a8 Deletion Preserves the Integrity of the Auditory Nerve (A) Typical auditory brainstem responses (ABRs) evoked by sound stimulation or by electrical stimulation applied via an electrode apposed onto the round-window membrane recorded in Slc17a8+/+ mice. The upper part of the figure shows superposed ABR traces evoked by a click presented at 50 dB SPL. Characteristic waves (I to IV) are clearly seen. The middle part of the figure shows ABR traces elicited by round-window electrical stimulation of 1 mA in the same animal. Note the similar pattern of ABR traces in both condition of stimulation. No electrophysiological response to sound or electrical pulses was recorded after round-window application of 10 μM Na+ channel blocker, TTX. (B) No acoustical ABR response was seen in _Slc17a8_−/− mice whatever the intensity of sound stimulation (top trace). In contrast, this knockout mice shows clear electrically evoked-ABRs (middle trace) that could be abolished after injection of 10 μM TTX into the cochlea (bottom trace). (C) Shown are input-output functions of ABRs wave I evoked by acoustical (gray symbols) or electrical (black symbols) stimulation in _Slc17a8_−/− and Slc17a8+/+ mice. Electrical ABRs amplitude is clearly reduced (black open circles) in _Slc17a8_−/− mice. Consistent with CAP data, no ABR could be evoked by sound stimulation in _Slc17a8_−/− mice (gray open circles). (D) The histogram represents the mean amplitude from 0 to 100 dB SPL sound stimulation and 0 to 2 mA electrical pulses for acoustically evoked ABRs (aABRs; gray bar) and electrically evoked ABRs (eABRs; black bar) in Slc17a8+/+ and in _Slc17a8_−/− mice, respectively. No response was recorded after cochlear injection of 10 μM TTX. Data presented are mean ± SEM, and n indicates the number of animal tested.
Similar articles
- VGLUT3-p.A211V variant fuses stereocilia bundles and elongates synaptic ribbons.
Joshi Y, Petit CP, Miot S, Guillet M, Sendin G, Bourien J, Wang J, Pujol R, El Mestikawy S, Puel JL, Nouvian R. Joshi Y, et al. J Physiol. 2021 Dec;599(24):5397-5416. doi: 10.1113/JP282181. Epub 2021 Dec 1. J Physiol. 2021. PMID: 34783032 Free PMC article. - Characterization of a Human Point Mutation of VGLUT3 (p.A211V) in the Rodent Brain Suggests a Nonuniform Distribution of the Transporter in Synaptic Vesicles.
Ramet L, Zimmermann J, Bersot T, Poirel O, De Gois S, Silm K, Sakae DY, Mansouri-Guilani N, Bourque MJ, Trudeau LE, Pietrancosta N, Daumas S, Bernard V, Rosenmund C, El Mestikawy S. Ramet L, et al. J Neurosci. 2017 Apr 12;37(15):4181-4199. doi: 10.1523/JNEUROSCI.0282-16.2017. Epub 2017 Mar 17. J Neurosci. 2017. PMID: 28314816 Free PMC article. - Screening of the SLC17A8 gene as a causative factor for autosomal dominant non-syndromic hearing loss in Koreans.
Ryu N, Sagong B, Park HJ, Kim MA, Lee KY, Choi JY, Kim UK. Ryu N, et al. BMC Med Genet. 2016 Jan 22;17:6. doi: 10.1186/s12881-016-0269-3. BMC Med Genet. 2016. PMID: 26797701 Free PMC article. - Review of hair cell synapse defects in sensorineural hearing impairment.
Moser T, Predoehl F, Starr A. Moser T, et al. Otol Neurotol. 2013 Aug;34(6):995-1004. doi: 10.1097/MAO.0b013e3182814d4a. Otol Neurotol. 2013. PMID: 23628789 Review. - Contribution of Vesicular Glutamate Transporters to Stress Response and Related Psychopathologies: Studies in VGluT3 Knockout Mice.
Horváth HR, Fazekas CL, Balázsfi D, Jain SK, Haller J, Zelena D. Horváth HR, et al. Cell Mol Neurobiol. 2018 Jan;38(1):37-52. doi: 10.1007/s10571-017-0528-7. Epub 2017 Aug 3. Cell Mol Neurobiol. 2018. PMID: 28776199 Review.
Cited by
- Molecular specializations underlying phenotypic differences in inner ear hair cells of zebrafish and mice.
Giffen KP, Liu H, Yamane KL, Li Y, Chen L, Kramer KL, Zallocchi M, He DZ. Giffen KP, et al. Front Neurol. 2024 Oct 17;15:1437558. doi: 10.3389/fneur.2024.1437558. eCollection 2024. Front Neurol. 2024. PMID: 39484049 Free PMC article. - Endolymphatic hydrops and cochlear synaptopathy after noise exposure are distinct sequelae of hair cell stereociliary bundle trauma.
Fong ML, Paik CB, Quiñones PM, Walker CB, Serafino MJ, Pan DW, Martinez E, Wang J, Phillips GW, Applegate BE, Gratton MA, Oghalai JS. Fong ML, et al. Sci Rep. 2024 Oct 27;14(1):25660. doi: 10.1038/s41598-024-77154-7. Sci Rep. 2024. PMID: 39465341 Free PMC article. - Brazilian Society of Otology task force - cochlear implant ‒ recommendations based on strength of evidence.
Tsuji RK, Hamerschmidt R, Lavinsky J, Felix F, Silva VAR. Tsuji RK, et al. Braz J Otorhinolaryngol. 2024 Sep 16;91(1):101512. doi: 10.1016/j.bjorl.2024.101512. Online ahead of print. Braz J Otorhinolaryngol. 2024. PMID: 39442262 Review. - Codon Usage Bias: A Potential Factor Affecting VGLUT Developmental Expression and Protein Evolution.
Zhao Y, Zhang Y, Feng J, He Z, Li T. Zhao Y, et al. Mol Neurobiol. 2024 Sep 21. doi: 10.1007/s12035-024-04426-8. Online ahead of print. Mol Neurobiol. 2024. PMID: 39305444 - Tissue engineering strategies for spiral ganglion neuron protection and regeneration.
Zhang B, Hu Y, Du H, Han S, Ren L, Cheng H, Wang Y, Gao X, Zheng S, Cui Q, Tian L, Liu T, Sun J, Chai R. Zhang B, et al. J Nanobiotechnology. 2024 Jul 31;22(1):458. doi: 10.1186/s12951-024-02742-8. J Nanobiotechnology. 2024. PMID: 39085923 Free PMC article. Review.
References
- Pleis J.R., Lethbridge-Cejku M. Summary health statistics for U.S. adults: National Health Interview Survey, 2005. Vital Health Stat. 2006;10:1–153. - PubMed
- Gates G.A., Couropmitree N.N., Myers R.H. Genetic associations in age-related hearing thresholds. Arch. Otolaryngol. Head Neck Surg. 1999;125:654–659. - PubMed
- Cruickshanks K.J., Wiley T.L., Tweed T.S., Klein B.E., Klein R., Mares-Perlman J.A., Nondahl D.M. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. The Epidemiology of Hearing Loss Study. Am. J. Epidemiol. 1998;148:879–886. - PubMed
- Brown S.D., Hardisty-Hughes R.E., Mburu P. Quiet as a mouse: Dissecting the molecular and genetic basis of hearing. Nat. Rev. Genet. 2008;9:277–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K23 DC000161/DC/NIDCD NIH HHS/United States
- R01 DC003544/DC/NIDCD NIH HHS/United States
- R01-DC003544/DC/NIDCD NIH HHS/United States
- K23 DC00161/DC/NIDCD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous