Antidepressant-like behavioral effects of IGF-I produced by enhanced serotonin transmission - PubMed (original) (raw)
Antidepressant-like behavioral effects of IGF-I produced by enhanced serotonin transmission
Brian A Hoshaw et al. Eur J Pharmacol. 2008.
Abstract
Previous research has suggested that mobilization of neurotrophic factors, such as insulin-like growth factor I (IGF-I), can be involved in the effects of antidepressant treatments. The current experiments showed that IGF-I leads to antidepressant-like effects in the modified rat forced swim test when tested 3 days, but not 1 day, after i.c.v. administration. These effects were sustained longer than the antidepressants paroxetine and desipramine. In addition, blockade of the IGF-I receptor with the IGF-I antagonist JB1 30 min before IGF-I administration prevented the antidepressant-like effects of IGF-I. However, when JB1 was administered 3 days after IGF-I administration and 30 min prior to testing, the antidepressant-like effects of IGF-I were still present suggesting that IGF-1 produces a long-term activation of neural systems involved in the antidepressant response. Because the pattern of antidepressant-like effects of IGF-I resembled those of selective serotonin reuptake inhibitors, the role of serotonin in the behavioral effects of IGF-I was studied. Depletion of serotonin, by the tryptophan hydroxylase inhibitor para-chlorophenylalanine, blocked the antidepressant-like effects of IGF-I. Administration of IGF-I increased basal serotonin levels in the ventral hippocampus and altered the effects of acute citalopram. IGF-I administration did not change hippocampal cell proliferation at the 3-day timepoint when behavioral effects were seen. In addition, IGF-I did not alter the expression of mRNA levels of tryptophan hydroxylase or SERT in the brain stem, or [3H] citalopram binding in the hippocampus or cortex. Thus, IGF-I administration initiates a long-lasting cascade of neurochemical effects involving increased serotonin levels that results in antidepressant-like behavioral effects.
Figures
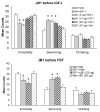
Fig. 1
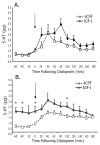
Blockade of antidepressant-like effects by the antagonist JB1given before but not after IGF-I. A, Rats were pretreated i.c.v. with either vehicle (aCSF) or varied doses of JB1 (5 (n = 5), 10 (n = 5), or 20 μg (n = 6); i.c.v.) 30 min before administration of either the vehicle or IGF-I (1 μg; i.c.v.). The FST session (5 min) was run 3 days later. Administration of vehicle-IGF-I (n = 12) decreased immobility and increased swimming behavior compared to the vehicle-vehicle group (n = 11). This effect was blocked by pretreatment with JB1 30 min before IGF-I, with the highest dose of JB1 blocking both the decrease in immobility and increase in swimming. ANOVAs revealed significant overall effects for immobility [F(5,39) = 7.33, P < .001] and swimming [F(5,39) = 6.25, P < .001] but not for climbing [F(5,39) = 1.25, P > .05].M B, Administration of JB1 3 days after IGF-I administration and 30 min before the FST, was not able to block the antidepressant-like effects of IGF-I. Rats were pretreated with either vehicle (aCSF) or IGF-I (1 μg) 24 h after the pretest session (15-min), and then administered either vehicle (aCSF) or JB1 (20 μg) 30 min before the FST session (5 min). Administration of IGF-I (n = 6) led to a significant decrease in immobility and a significant increase in swimming compared to the vehicle-vehicle group (n = 7), but administration of JB1 30 min before the FST (n = 7) did not block those behavioral effects. There were no significant differences between the vehicle-JB1 group (n = 6) and the vehicle-vehicle group. Two-factor ANOVA revealed that there was a significant effect for drug treatment (IGF-I vs. aCSF) in immobility [F(1,22) = 19.32, P < .001] and swimming [F(1,22) = 33.89, P < .0001], but no effect for pretreatment group (JB1 vs. aCSF) for either immobility [F(5,39) = 0.58, P > .05] or swimming [F(5,39) = 0.23, P > .05]. Values represent mean ± 1 S.E.M. * indicates P < .05 compared with the vehicle-vehicle group.
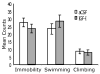
Fig. 2
IGF-I did not fully produce antidepressant-like effects in the FST when tested 1 day after administration. IGF-I (1 μg; n = 7) or vehicle (aCSF; n = 7) was administered 24 h before the FST pretest (15-min), and the 5-min FST session was run 1 day after injection. Values represent mean ± 1 S.E.M.
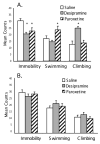
Fig. 3
Effects of antidepressants in the modified FST, either the SSRI paroxetine or the selective norepinephrine reuptake inhibitor desipramine,. A, Rats were tested in the FST 1 h after the last of 3 injections with either paroxetine (20 mg/kg), desipramine (20 mg/kg) or saline (n=10 each). One-factor ANOVA revealed significant effects for immobility [F(2,21) = 5.96, P < .001], swimming [F(2,21) = 8.35, P < .003], and climbing [F(2,21) = 6.45, P < .007]. Both drugs showed antidepressant-like effects. The SSRI paroxetine decreased immobility and increased swimming, while desipramine decreased immobility and increased climbing. B, Neither antidepressant was active in the FST when the rats were tested 3 days after the last injection. Values represent mean ± 1 S.E.M. * indicates P < .05 compared with saline.
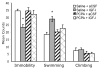
Fig. 4
Depletion of serotonin blocked the antidepressant-like effects of IGF-I in the FST. Serotonin synthesis was inhibited by administering the tryptophan hydroxylase inhibitor PCPA (300 mg/kg; i.p.) 24 h before the first 15-min session. Vehicle or IGF-I (1 μg) was then administered 24 h later, and the rats were tested in the second session of the FST 3 days later, or 96 h after PCPA pretreatment. IGF-I (n = 9) produced a significant decrease in immobility and increase in swimming compared to the saline-vehicle group (n = 9). Pretreatment with PCPA before vehicle (n = 11) had no effect on any of the three behaviors compared to the saline-vehicle group. However, PCPA pretreatment blocked the antidepressant-like effects of IGF-I (n = 11). Therefore, serotonin transmission appears to be necessary for the antidepressant-like effects of IGF-I. Values represent mean ± 1 S.E.M. * indicates P < .05 compared with the saline-vehicle group.
Fig. 5
IGF-I administration increased basal serotonin levels in the ventral hippocampus 3 days, but not 1 day, after administration as measured using in vivo microdialysis. Values represent mean (pg) ± 1 S.E.M. * indicates P < .05 compared with aCSF control. A, Hippocampal serotonin levels measured 1 day after IGF-I (1 μg; n = 6) administration. Four 20-min samples were collected as a baseline measure before the systemic injection of citalopram (2.5 mg/kg; i.p.). IGF-1 did not affect basal serotonin levels compared with vehicle (aCSF; n = 4). There was a trend towards an increase in the percentage change in serotonin levels after citalopram administration (P = .07). B, Hippocampal serotonin levels measured 3 days after administration of either vehicle (aCSF; n = 6) or IGF-I (1 μg; n = 5) administration. The IGF-I-pretreated rats had significantly higher basal serotonin levels (P < .02). Although the cumulative output after citalopram injection was smaller in rats treated with IGF-1, the difference was not significant (P = .09). The time course for the ability of IGF-I to increase serotonin levels in the ventral hippocampus coincides with the time course for the appearance of behavioral effects in the FST.
Fig. 6
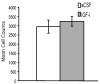
Cell proliferation in the dentate gyrus of the hippocampus was not affected 3 days after IGF-I administration. The number of cells labeled with BrdU in the subgranular zone of the dentate gyrus were counted and compared to vehicle controls. The thymidine analog BrdU (100 mg/kg, i.p.) was injected 3 days after a single administration of either vehicle or IGF-I (1 μg; n = 7 each). Rats were perfused 2 h later and immunohistochemistry for BrdU performed to visualize labeled cells on the sectioned tissue. Values represent mean ± 1 S.E.M.
Similar articles
- Sequential changes in BDNF mRNA expression and synaptic levels of AMPA receptor subunits in rat hippocampus after chronic antidepressant treatment.
Martínez-Turrillas R, Del Río J, Frechilla D. Martínez-Turrillas R, et al. Neuropharmacology. 2005 Dec;49(8):1178-88. doi: 10.1016/j.neuropharm.2005.07.006. Epub 2005 Sep 6. Neuropharmacology. 2005. PMID: 16143352 - Association of changes in norepinephrine and serotonin transporter expression with the long-term behavioral effects of antidepressant drugs.
Zhao Z, Zhang HT, Bootzin E, Millan MJ, O'Donnell JM. Zhao Z, et al. Neuropsychopharmacology. 2009 May;34(6):1467-81. doi: 10.1038/npp.2008.183. Epub 2008 Oct 15. Neuropsychopharmacology. 2009. PMID: 18923402 Free PMC article. - Effect of antidepressant drugs on 6-OHDA-treated mice in the FST.
Chenu F, Dailly E, Bourin M. Chenu F, et al. Eur Neuropsychopharmacol. 2007 Feb;17(3):187-93. doi: 10.1016/j.euroneuro.2006.04.006. Epub 2006 Jun 6. Eur Neuropsychopharmacol. 2007. PMID: 16757155 - Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.
Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Repérant C, Guilloux JP, Coudoré F, Hen R, Gardier AM. Deltheil T, et al. Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review. - Norepinephrine involvement in antidepressant action.
Frazer A. Frazer A. J Clin Psychiatry. 2000;61 Suppl 10:25-30. J Clin Psychiatry. 2000. PMID: 10910014 Review.
Cited by
- Boosting Neurogenesis in the Adult Hippocampus Using Antidepressants and Mesenchymal Stem Cells.
Kot M, Neglur PK, Pietraszewska A, Buzanska L. Kot M, et al. Cells. 2022 Oct 14;11(20):3234. doi: 10.3390/cells11203234. Cells. 2022. PMID: 36291101 Free PMC article. Review. - Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice.
Park SE, Dantzer R, Kelley KW, McCusker RH. Park SE, et al. J Neuroinflammation. 2011 Feb 9;8:12. doi: 10.1186/1742-2094-8-12. J Neuroinflammation. 2011. PMID: 21306618 Free PMC article. - Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia, gut dysbiosis, and metabolic and functional brain regional deficits.
Higarza SG, Arboleya S, Gueimonde M, Gómez-Lázaro E, Arias JL, Arias N. Higarza SG, et al. PLoS One. 2019 Sep 20;14(9):e0223019. doi: 10.1371/journal.pone.0223019. eCollection 2019. PLoS One. 2019. PMID: 31539420 Free PMC article. - Enriched early life experiences reduce adult anxiety-like behavior in rats: a role for insulin-like growth factor 1.
Baldini S, Restani L, Baroncelli L, Coltelli M, Franco R, Cenni MC, Maffei L, Berardi N. Baldini S, et al. J Neurosci. 2013 Jul 10;33(28):11715-23. doi: 10.1523/JNEUROSCI.3541-12.2013. J Neurosci. 2013. PMID: 23843538 Free PMC article. - Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression.
Levy MJF, Boulle F, Steinbusch HW, van den Hove DLA, Kenis G, Lanfumey L. Levy MJF, et al. Psychopharmacology (Berl). 2018 Aug;235(8):2195-2220. doi: 10.1007/s00213-018-4950-4. Epub 2018 Jun 30. Psychopharmacology (Berl). 2018. PMID: 29961124 Free PMC article. Review.
References
- Aguado F, Rodrigo J, Cacicedo L, Mellstrom B. Distribution of insulin-like growth factor-I receptor mRNA in rat brain. Regulation in the hypothalamo-neurohypophysial system. J Mol Endocrin. 1993;11:231–239. - PubMed
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. - PubMed
- Altar CA. Neurotrophins and depression. Trends Pharmacol Sci. 1999;20:59–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 MH014654-30/MH/NIMH NIH HHS/United States
- U01 MH072832-02/MH/NIMH NIH HHS/United States
- T32 MH014654/MH/NIMH NIH HHS/United States
- MH72832/MH/NIMH NIH HHS/United States
- U01 MH072832/MH/NIMH NIH HHS/United States
- U01 MH072832-01/MH/NIMH NIH HHS/United States
- MH14654/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous