Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease - PubMed (original) (raw)
Review
Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease
Ilya Bezprozvanny et al. Trends Neurosci. 2008 Sep.
Abstract
Perturbed neuronal Ca(2+) homeostasis is implicated in age-related cognitive impairment and Alzheimer's disease (AD). With advancing age, neurons encounter increased oxidative stress and impaired energy metabolism, which compromise the function of proteins that control membrane excitability and subcellular Ca(2+) dynamics. Toxic forms of amyloid beta-peptide (Abeta) can induce Ca(2+) influx into neurons by inducing membrane-associated oxidative stress or by forming an oligomeric pore in the membrane, thereby rendering neurons vulnerable to excitotoxicity and apoptosis. AD-causing mutations in the beta-amyloid precursor protein and presenilins can compromise these normal proteins in the plasma membrane and endoplasmic reticulum, respectively. Emerging knowledge of the actions of Ca(2+) upstream and downstream of Abeta provides opportunities to develop novel preventative and therapeutic interventions for AD.
Figures
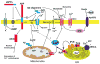
Figure 1
Mechanisms that may result in perturbed neuronal Ca2+ homeostasis in Alzheimer’s disease. Sequential cleavages of the β-amyloid precursor protein (APP) by β-secretase (β) and γ-secretase (γ) generates the amyloid β-peptide (Aβ). Aβ forms oligomers which can insert into the plasma membrane and form pores through which Ca2+ passes into the cytoplasm. The interaction of Aβ with the plasma membrane may be facilitated by binding to phosphatidylserine (PtdS); age/AD-related mitochondrial impairment (ATP depletion) may trigger flipping of PtdS from the inner portion of the plasma membrane to the cell surface. The PtdS flipping may also result from Ca2+ influx or release from the endoplasmic reticulum (ER) or mitochondria which can activate a phospholipid scramblase (PLSCR1). Aβ can also interact with Fe2+ and Cu+ to generate hydrogen peroxide and hydroxyl radical (OH.) resulting in membrane lipid peroxidation which generates toxic aldehydes that impair the function of membrane ion-motive ATPases (Na+ and Ca2+ pumps). As a result the membrane becomes depolarized and glutamate receptor channels (NMDAR, N-methyl-D-aspartate receptor) and voltage-dependent Ca2+ channels (VDCC) open and flux toxic amounts of Ca2+ into the cytoplasm. In addition, Aβ acts on mitochondria (either directly or indirectly by causing elevated cytoplasmic Ca2+ levels and oxidative stress) to cause increased free radical (superoxide anion radical; O2−) production, Ca2+ overload and decreased ATP production. Amyloidogenic processing also generates an intracellular APP domain (AICD) which can translocate to the nucleus and modify gene transcription in ways that perturb Ca2+ homeostasis. Studies of the presenilins (PS) have implicated ER Ca2+ mishandling in AD. PS functions as an ER Ca2+ leak channel and FAD PS mutations impair this Ca2+ leak channel function resulting in excessive accumulation of Ca2+ in the ER and, as a consequence, enhances Ca2+ release through ryanodine receptor (RyR) and IP3 receptor (IP3R) channels. There is also evidence that PS can interact directly or indirectly with RyR and SERCA (smooth endoplasmic reticulum Ca2+-ATPase) to alter ER Ca2+ release and uptake. Interaction of the protein reelin with the apolipoprotein E receptor (ApoER2) enhances Ca2+ influx through NMDA receptor channels by a mechanism involving a src family tyrosine kinsase (SFk); ApoE can block this effect of reelin. Finally, amyloidogenic APP processing may prevent α-secretase (α) cleavage of APP which would otherwise generate a secreted form of APP (sAPPα). sAPPα is normally produced in response to synaptic activity and is known to activate a signaling pathway involving cyclic guanosine monophosphate (cGMP) that activates K+ channels, thereby hyperpolarizing the membrane and reducing Ca2+ influx. The mechanisms illustrated here include abundant targets for therapeutic intervention (see also Fig. 3) and one drug that targets the NMDA receptor (memantine) is currently used to treat AD patients.
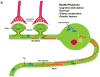
Figure 2
Interactions of Ca2+ and the neuronal cytoskeleton in AD pathogenesis. A. In healthy neurons the axon contains relatively high amounts of microtubules which are stabilized by the protein tau. Microtubule dynamics in axons play pivotal roles in organellar (mitochondria, for example) and protein transport to presynaptic axon terminals. Dendrites receive synaptic inputs in postsynaptic structures called spines whose shape is controlled by actin filaments and various scaffolding proteins. Ca2+ influx during synaptic activity modifies the dynamics of actin and microtubules in ways that allow the neuron to adapt to environmental demands. B. During the course of AD tau becomes hyperphosphorylated and dissociates from microtubules which then depolymerize. The hyperphosphorylated tau self-aggregates and accumulates in the cell body where it forms paired-helical filaments (neurofibrillary tangles). As a consequence of accumulation of Aβ at synapses, Ca2+ regulation is impaired, the dendritic spines atrophy and the underlying cytoskeletal scaffold is disrupted resulting in synaptic degeneration. In these ways, cytoskeletal abnormalities underlie cognitive impairment in AD.
Figure 2
Interactions of Ca2+ and the neuronal cytoskeleton in AD pathogenesis. A. In healthy neurons the axon contains relatively high amounts of microtubules which are stabilized by the protein tau. Microtubule dynamics in axons play pivotal roles in organellar (mitochondria, for example) and protein transport to presynaptic axon terminals. Dendrites receive synaptic inputs in postsynaptic structures called spines whose shape is controlled by actin filaments and various scaffolding proteins. Ca2+ influx during synaptic activity modifies the dynamics of actin and microtubules in ways that allow the neuron to adapt to environmental demands. B. During the course of AD tau becomes hyperphosphorylated and dissociates from microtubules which then depolymerize. The hyperphosphorylated tau self-aggregates and accumulates in the cell body where it forms paired-helical filaments (neurofibrillary tangles). As a consequence of accumulation of Aβ at synapses, Ca2+ regulation is impaired, the dendritic spines atrophy and the underlying cytoskeletal scaffold is disrupted resulting in synaptic degeneration. In these ways, cytoskeletal abnormalities underlie cognitive impairment in AD.
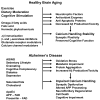
Figure 3
Plausible mechanisms for healthy brain aging and AD. Healthy brain aging can be promoted by regular exercise, moderation in caloric intake and engaging in intellectually challenging activities. These lifestyle factors may stabilize neuronal Ca2+ homeostasis and counteract aging by inducing the production of neurotrophic factors, cellular antioxidant defense systems and cell survival-promoting proteins. Specific dietary factors may also reduce the risk of AD including omega-3 fatty acids, folic acid and possibly phytochemicals106. By stabilizing neuronal Ca2+ homeostasis the healthy lifestyle and dietary factors can preserve synaptic and cognitive function during aging. Lifestyle factors that may accelerate aging and AD pathogenesis include lack of exercise, overeating and intellectually unchallenging daily activities. The risk of deleloping AD is reduced by the presence of ApoE2 allele and increased by ApoE4. Genetic mutations in presenilins and APP lead to early-onset familial AD. Two disorders that increase the risk for AD are diabetes and depression. Diets high in saturated fats and cholesterol may also adversely affect the brain by increasing oxidative stress and perturbing cell membranes. As a result of the aging process, and genetic and lifestyle factors, neurons suffer from increased oxidative stress, metabolic impairment and the accumulation of aggregated proteins including Aβ and tau. All of the latter alterations perturb neuronal Ca2+ homeostasis resulting in synaptic dysfunction, amyloidogenic APP processing, tau pathology, neuronal death and cognitive dysfunction. Treatments that target Aβ, including immunization and β- and γ-secretase inhibitors may stabilize neuronal Ca2+ homeostasis by preventing the adverse actions of Aβ on membranes and associated Ca2+-regulating proteins (see Fig. 1). Drugs that modulate ion channels involved in Ca2+ influx and release from internal organelles have the potential to counteract neurodegenerative processes in AD.
Similar articles
- Aberrant subcellular neuronal calcium regulation in aging and Alzheimer's disease.
Camandola S, Mattson MP. Camandola S, et al. Biochim Biophys Acta. 2011 May;1813(5):965-73. doi: 10.1016/j.bbamcr.2010.10.005. Epub 2010 Oct 13. Biochim Biophys Acta. 2011. PMID: 20950656 Free PMC article. Review. - Dysregulation of cellular calcium homeostasis in Alzheimer's disease: bad genes and bad habits.
Mattson MP, Chan SL. Mattson MP, et al. J Mol Neurosci. 2001 Oct;17(2):205-24. doi: 10.1385/JMN:17:2:205. J Mol Neurosci. 2001. PMID: 11816794 Review. - Presenilins as endoplasmic reticulum calcium leak channels and Alzheimer's disease pathogenesis.
Supnet C, Bezprozvanny I. Supnet C, et al. Sci China Life Sci. 2011 Aug;54(8):744-51. doi: 10.1007/s11427-011-4201-y. Epub 2011 Jul 24. Sci China Life Sci. 2011. PMID: 21786197 Review. - Presenilins as Drug Targets for Alzheimer's Disease-Recent Insights from Cell Biology and Electrophysiology as Novel Opportunities in Drug Development.
Duncan RS, Song B, Koulen P. Duncan RS, et al. Int J Mol Sci. 2018 May 31;19(6):1621. doi: 10.3390/ijms19061621. Int J Mol Sci. 2018. PMID: 29857474 Free PMC article. Review. - Neuronal and glial calcium signaling in Alzheimer's disease.
Mattson MP, Chan SL. Mattson MP, et al. Cell Calcium. 2003 Oct-Nov;34(4-5):385-97. doi: 10.1016/s0143-4160(03)00128-3. Cell Calcium. 2003. PMID: 12909083 Review.
Cited by
- Long-term dantrolene treatment reduced intraneuronal amyloid in aged Alzheimer triple transgenic mice.
Wu Z, Yang B, Liu C, Liang G, Eckenhoff MF, Liu W, Pickup S, Meng Q, Tian Y, Li S, Wei H. Wu Z, et al. Alzheimer Dis Assoc Disord. 2015 Jul-Sep;29(3):184-191. doi: 10.1097/WAD.0000000000000075. Alzheimer Dis Assoc Disord. 2015. PMID: 25650693 Free PMC article. - Determining the Roles of Inositol Trisphosphate Receptors in Neurodegeneration: Interdisciplinary Perspectives on a Complex Topic.
Takada SH, Ikebara JM, de Sousa E, Cardoso DS, Resende RR, Ulrich H, Rückl M, Rüdiger S, Kihara AH. Takada SH, et al. Mol Neurobiol. 2017 Nov;54(9):6870-6884. doi: 10.1007/s12035-016-0205-8. Epub 2016 Oct 22. Mol Neurobiol. 2017. PMID: 27771899 Review. - Upregulated function of mitochondria-associated ER membranes in Alzheimer disease.
Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Area-Gomez E, et al. EMBO J. 2012 Nov 5;31(21):4106-23. doi: 10.1038/emboj.2012.202. Epub 2012 Aug 14. EMBO J. 2012. PMID: 22892566 Free PMC article. - Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia.
Berridge MJ. Berridge MJ. Prion. 2013 Jan-Feb;7(1):2-13. doi: 10.4161/pri.21767. Epub 2012 Aug 16. Prion. 2013. PMID: 22895098 Free PMC article. Review.
References
- Berridge MJ, Bootman MD, Lipp P. Calcium - a life and death signal. Nature. 1998;395:645–648. - PubMed
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038082/NS/NINDS NIH HHS/United States
- R01 NS056224/NS/NINDS NIH HHS/United States
- Z01 AG000312-07/ImNIH/Intramural NIH HHS/United States
- R01 NS38082/NS/NINDS NIH HHS/United States
- R01 NS038082-07/NS/NINDS NIH HHS/United States
- R01 NS056224-02/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous