Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy - PubMed (original) (raw)
Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy
C R Houser et al. Neuroscience. 2008.
Abstract
Extracellular signal-regulated kinase (ERK) is highly sensitive to regulation by neuronal activity and is critically involved in several forms of synaptic plasticity. These features suggested that alterations in ERK signaling might occur in epilepsy. Previous studies have described increased ERK phosphorylation immediately after the induction of severe seizures, but patterns of ERK activation in epileptic animals during the chronic period have not been determined. Thus, the localization and abundance of phosphorylated extracellular signal-regulated kinase (pERK) were examined in a pilocarpine model of recurrent seizures in C57BL/6 mice during the seizure-free period and at short intervals after spontaneous seizures. Immunolabeling of pERK in control animals revealed an abundance of distinctly-labeled neurons within the hippocampal formation. However, in pilocarpine-treated mice during the seizure-free period, the numbers of pERK-labeled neurons were substantially decreased throughout much of the hippocampal formation. Double labeling with a general neuronal marker suggested that the decrease in pERK-labeled neurons was not due primarily to cell loss. The decreased ERK phosphorylation in seizure-prone animals was interpreted as a compensatory response to increased neuronal excitability within the network. Nevertheless, striking increases in pERK labeling occurred at the time of spontaneous seizures and were evident in large populations of neurons at very short intervals (as early as 2 min) after detection of a behavioral seizure. These findings suggest that increased pERK labeling could be one of the earliest immunohistochemical indicators of neurons that are activated at the time of a spontaneous seizure.
Figures
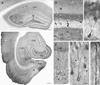
Fig. 1. Phospho-ERK (pERK) labeling in the hippocampal formation of control mice
(A) In a coronal section, darkly-labeled cell bodies are scattered throughout the lightly labeled granule cell layer (G) of the dentate gyrus and pyramidal cell layer (P) of the hippocampus (CA1–CA3). Labeled dendrites contribute to light labeling in the adjacent dendritic layers. Moderate levels of diffuse labeling are present in the inner molecular layer (M) and in the mossy fiber path within the hilus and s. lucidum of CA3 (arrow). (B) Small numbers of neurons in the granule cell layer (G) of the dentate gyrus are strongly labeled, and their dendrites extend into the molecular layer (M). A few moderately-labeled neurons are evident in the hilus (H). (C) Labeling extends throughout the cytoplasm of the cell body, branching dendrite and axon (arrow) of a granule cell. Labeled terminal-like structures form a band in the inner molecular layer (M). (D) In a horizontal section of the ventral hippocampus, strong labeling of scattered neurons within the principal cell layers of the dentate gyrus (DG) and hippocampus (CA1–CA3) closely resembles the patterns in the dorsal hippocampus. Numerous pERK-labeled neurons are also present in the subiculum (S) and a transition zone (arrow) between the parasubiculum (PaS) and the medial borders of the entorhinal cortex (ER). Relatively few labeled neurons are found in the presubiculum (PrS). A laminar pattern of labeled cells is evident in the entorhinal cortex whereas a more homogeneous distribution is present in the adjacent perirhinal cortex (PR). Few labeled cell bodies are evident in the most medial region of the entorhinal cortex (arrowhead). (E) Higher magnification of CA1 reveals pERK labeling of cell bodies in the pyramidal cell layer (P). Numerous labeled apical dendrites extend through stratum radiatum (R) and branch at the border of lacunosum-moleculare (L–M). (F) A labeled pyramidal cell (arrow) in CA1 exhibits strong labeling in the cytoplasm of the cell body and ascending dendrite, with only light labeling of the nucleus. The pERK-labeled cell bodies contrast sharply with unlabeled cell bodies in the field (arrowheads). (G) Numerous labeled spines (examples at arrows) are evident along an apical dendrite of a CA1 pyramidal cell. Scale bars = 200 µm in A,D; 20 µm in B; 10 µm in C,F,G; 50 µm in E.
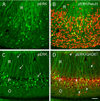
Fig. 2. Comparisons of pERK (A–D, green), NeuN (B, red) and GAD67 (D, red) labeling in the subiculum (A–B) and CA1 (C–D)
(A) pERK-labeled neurons are evident throughout the cell body layer of the subiculum, and their labeled dendrites branch extensively in stratum radiatum (R). (B) Neurons that are double-labeled for pERK and NeuN (yellow) constitute a relatively small subset of the much larger population of NeuN-labeled neurons (red) in the subiculum. (C) Cell bodies of pERK-labeled neurons are concentrated in the pyramidal cell layer (P) of CA1, and their apical dendrites (examples at arrows) extend relatively unbranched through stratum radiatum (R). Few pERK-labeled neurons are evident in stratum oriens (O). (D) Many GAD67-labeled neurons (red, examples at arrowheads) in strata oriens (O), pyramidale (P) and radiatum (R) are not co-localized with pERK (green). Much of the GAD67 labeling in stratum pyramidale represents the rich plexus of GABAergic terminals in this region. Scale bar (in D) = 50 µm in A–D.
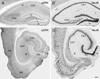
Fig. 3. Phospho-ERK (pERK) (A,C) and NeuN (B,D) labeling in the dorsal (A,B; coronal) and ventral (C,D; horizontal) hippocampal formation of pilocarpine-treated mice during the chronic period
(A,C) pERK labeling is greatly reduced in most regions of the hippocampal formation and adjacent cortex compared to that in control animals (compare with Fig. 1, A,D). Numbers of strongly-labeled neurons in the granule cell layer (G) of the dentate gyrus (DG) and pyramidal cell layer (P) of the hippocampus (CA1–CA3) are substantially reduced, and the labeled neurons that can be detected are lightly labeled. (B,D) NeuN labeling in the same animals demonstrates the preservation of many neurons throughout the hippocampal formation, and thus cell loss is unlikely to account for the decrease in pERK labeling. Regions with the greatest cell loss are the hilus (H) and some regions of CA3. Additional labels in panels C,D: Entorhinal cortex (ER), perirhinal cortex (PR), subiculum (S). Scale bar (in D) = 200 µm in A–D.
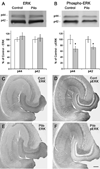
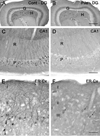
Fig. 4. Comparisons of ERK (A,C,E) and phospho-ERK (B,D,F) in control and pilocarpine-treated mice during the chronic period
In A,B, representative Western blots are shown above graphs of summary quantification (Control, white bars; Pilocarpine-treated, grey bars). (A) Pilocarpine-treated mice show no significant difference in either the p44 or p42 band of total ERK in the hippocampus when compared to control mice. (B) Pilocarpine-treated mice show a decrease in both the p44 and p42 bands of phospho-ERK in the hippocampus when compared to control mice. Error bars for A,B indicate +/− SEM; n = 6 pilocarpine-treated mice and 6 age-matched control mice; asterisks indicate significant differences (p < 0.05). (C,E) Immunohistochemical labeling of total ERK was similar in control and pilocarpine-treated mice. (D,F) Immunohistochemical labeling of pERK, in sections from the same animals used for ERK labeling, demonstrate substantial decreases in intensely-labeled neurons in the pilocarpine-treated animal when compared to the control. Scale bar (in F) = 200 µm in C–F.
Fig. 5. Phospho-ERK labeling in control (A,C,E) and pilocarpine-treated animals (B,D,F) during the chronic stage
(A,B) In the dentate gyrus (DG), the number of labeled neurons in the granule cell layer (G) is substantially reduced in the pilocarpine-treated mice. Numbers of pERK-labeled neurons in the hilus (H) are also reduced, but cell loss in this region could account for some of this loss. (C,D) In CA1, fewer strongly-labeled cell bodies in stratum pyramidale (P) and dendrites in stratum radiatum (R) are evident in the epileptic animals, and neurons that are labeled exhibit lighter labeling than those in control animals. (E,F) In the entorhinal cortex (ER Cx), pERK-labeled cell bodies are normally concentrated in layer III, and labeled dendrites of these neurons extend into layers I and II. In the pilocarpine-treated animals, pERK labeling of cell bodies and dendrites is greatly decreased. Some lightly labeled neurons (arrows) are evident, suggesting that the neurons persist but have lost their extensive labeling. Scale bars (in B,D,F) = 200 µm in A,B; 100 µm in C,D; 50 µm in E,F.
Fig. 6. Densities of pERK-labeled cell bodies in control and pilocarpine-treated mice
In the principal cell layer of five hippocampal regions, the densities of pERK-labeled neurons were highest in CA1 and CA2 and lowest in CA3 and the granule cell layer of the dentate gyrus (DG). Statistically significant decreases in densities of labeled neurons were found in all regions examined in the pilocarpine-treated mice (p< 0.001).
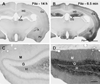
Fig. 7. Comparisons of pERK labeling in pilocarpine-treated mice at 14 h (A,C) and 6.5 min (B,D) after detection of a spontaneous seizure
(A,B) Basal levels of pERK labeling are low during the interictal period but increase dramatically following a spontaneous behavioral seizure. Increased pERK labeling is evident in the dentate gyrus (DG), amygdaloid region (A), piriform cortex (Pir), and other cortical regions (Cx). Only a slight increase is noted in the hippocampus (HC) in this animal. (C,D) In the dentate gyrus during the interictal period, very few labeled cell bodies are evident in the granule cell layer (G). In contrast, at 6.5 min after detection of a spontaneous seizure, a high proportion of somata are labeled in the granule cell layer (G). pERK labeling is also increased in granule cell dendrites throughout the molecular layer (M) and in mossy fibers within the hilus (H). Scale bars (in B,D) = 1 mm in A,B; 100 µm in C,D.
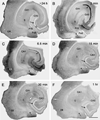
Fig. 8. Phospho-ERK labeling in the hippocampus of pilocarpine-treated mice at different time points after spontaneous seizures
(A) In a mouse that was seizure-free for more than 24 h prior to perfusion, the number of pERK-labeled neurons throughout the hippocampal formation is low, and the granule cell layer (G) is virtually free of labeling (arrow). (B) In a mouse at 2 min after detection of a spontaneous seizure, intense pERK labeling is evident in some regions of the granule cell layer (arrow) and in the mossy fiber path (arrowhead). Numerous labeled cell bodies are evident in several regions of the entorhinal cortex (ER) and in a transition zone (asterisk) between the parasubiculum (PaS) and entorhinal cortex. (C) In a mouse at 6.5 min following seizure detection, strong pERK labeling extends throughout much of the granule cell layer (arrow) and the mossy fiber path (arrowhead). Labeled neurons are evident in the subiculum (S), and strong, diffuse pERK labeling is present in the transition zone (asterisk) and regions of the entorhinal cortex (ER). (D) In a mouse at 15 min after a spontaneous seizure, pERK labeling is very low in the granule cell layer (arrow) where it resembles the labeling during the interictal period (Panel A). Scattered labeled neurons are evident in most regions of the hippocampal formation, but the strong diffuse labeling is less than in the mouse at the earlier time point (Panel C). (E) In a mouse at 30 min after a spontaneous seizure, pERK labeling has decreased further throughout the hippocampal formation. (F) At 1 h after a spontaneous seizure, the number of pERK-labeled neurons is greatly decreased, and the labeling resembles that of a mouse that had not experienced recent spontaneous seizures (compare with Panel A). Scale bar (in F) = 500 µm in A–F.
Fig. 9. Comparison of pERK (A,C,E) and Fos (B,D,F) labeling in the hippocampal formation at progressively longer times after spontaneous seizures
(A,B) At 2 min after a spontaneous seizure, strong pERK labeling is evident in the granule cell layer (arrow) and mossy fiber path (arrowhead). Dendritic layers of the dentate gyrus (DG) and hippocampus (HC) are also labeled. At this early time point, no labeling for Fos is evident. (C,D) At 15 min after a spontaneous seizure, pERK labeling is low in the granule cell layer (arrow), but remains high in the mossy fiber path (arrowhead). Fos labeling is present within numerous granule cells throughout the granule cell layer (arrow). (E,F) By 30 min after a spontaneous seizure, moderate to low levels of pERK labeling are evident in most regions of the dentate gyrus and hippocampus, and the granule cell layer is unlabeled (arrow). Strong Fos labeling is present in the granule cell layer (arrow) at this time. Across time points, alterations in pERK labeling appear to occur rapidly, consistent with the phosphorylation process, and are evident in cell bodies, dendrites and axons. In contrast, changes in Fos labeling occur later and are restricted to neuronal nuclei. Scale bar (in F) = 200 µm in A–F.
Similar articles
- Activation of ERK by spontaneous seizures in neural progenitors of the dentate gyrus in a mouse model of epilepsy.
Li Y, Peng Z, Xiao B, Houser CR. Li Y, et al. Exp Neurol. 2010 Jul;224(1):133-45. doi: 10.1016/j.expneurol.2010.03.003. Epub 2010 Mar 10. Exp Neurol. 2010. PMID: 20226181 Free PMC article. - Functional role of mGluR1 and mGluR4 in pilocarpine-induced temporal lobe epilepsy.
Pitsch J, Schoch S, Gueler N, Flor PJ, van der Putten H, Becker AJ. Pitsch J, et al. Neurobiol Dis. 2007 Jun;26(3):623-33. doi: 10.1016/j.nbd.2007.03.003. Epub 2007 Mar 16. Neurobiol Dis. 2007. PMID: 17446080 - Vulnerability and plasticity of the GABA system in the pilocarpine model of spontaneous recurrent seizures.
Houser CR, Esclapez M. Houser CR, et al. Epilepsy Res. 1996 Dec;26(1):207-18. doi: 10.1016/s0920-1211(96)00054-x. Epilepsy Res. 1996. PMID: 8985701 - Insulin growth factor-1 (IGF-1) enhances hippocampal excitatory and seizure activity through IGF-1 receptor-mediated mechanisms in the epileptic brain.
Jiang G, Wang W, Cao Q, Gu J, Mi X, Wang K, Chen G, Wang X. Jiang G, et al. Clin Sci (Lond). 2015 Dec;129(12):1047-60. doi: 10.1042/CS20150312. Epub 2015 Aug 18. Clin Sci (Lond). 2015. PMID: 26286172 - Circadian clustering of spontaneous epileptic seizures emerges after pilocarpine-induced status epilepticus.
Pitsch J, Becker AJ, Schoch S, Müller JA, de Curtis M, Gnatkovsky V. Pitsch J, et al. Epilepsia. 2017 Jul;58(7):1159-1171. doi: 10.1111/epi.13795. Epub 2017 May 24. Epilepsia. 2017. PMID: 28542864
Cited by
- Neuroanatomical clues to altered neuronal activity in epilepsy: from ultrastructure to signaling pathways of dentate granule cells.
Houser CR, Zhang N, Peng Z, Huang CS, Cetina Y. Houser CR, et al. Epilepsia. 2012 Jun;53 Suppl 1(0 1):67-77. doi: 10.1111/j.1528-1167.2012.03477.x. Epilepsia. 2012. PMID: 22612811 Free PMC article. Review. - Time-dependent modulation of mitogen activated protein kinases and AKT in rat hippocampus and cortex in the pilocarpine model of epilepsy.
Lopes MW, Soares FM, de Mello N, Nunes JC, de Cordova FM, Walz R, Leal RB. Lopes MW, et al. Neurochem Res. 2012 Sep;37(9):1868-78. doi: 10.1007/s11064-012-0797-y. Epub 2012 May 22. Neurochem Res. 2012. PMID: 22614924 - Neurosteroid regulation of GABAA receptors: A role in catamenial epilepsy.
Joshi S, Kapur J. Joshi S, et al. Brain Res. 2019 Jan 15;1703:31-40. doi: 10.1016/j.brainres.2018.02.031. Epub 2018 Feb 23. Brain Res. 2019. PMID: 29481795 Free PMC article. Review. - Frontier of epilepsy research - mTOR signaling pathway.
Cho CH. Cho CH. Exp Mol Med. 2011 May 31;43(5):231-74. doi: 10.3858/emm.2011.43.5.032. Exp Mol Med. 2011. PMID: 21467839 Free PMC article. Review. - Epilepsy, Antiepileptic Drugs, and Aggression: An Evidence-Based Review.
Brodie MJ, Besag F, Ettinger AB, Mula M, Gobbi G, Comai S, Aldenkamp AP, Steinhoff BJ. Brodie MJ, et al. Pharmacol Rev. 2016 Jul;68(3):563-602. doi: 10.1124/pr.115.012021. Pharmacol Rev. 2016. PMID: 27255267 Free PMC article. Review.
References
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. - PubMed
- Arabadzisz D, Antal K, Parpan F, Emri Z, Fritschy JM. Epileptogenesis and chronic seizures in a mouse model of temporal lobe epilepsy are associated with distinct EEG patterns and selective neurochemical alterations in the contralateral hippocampus. Exp Neurol. 2005;194:76–90. - PubMed
- Baraban JM, Fiore RS, Sanghera JS, Paddon HB, Pelech SL. Identification of p42 mitogen-activated protein kinase as a tyrosine kinase substrate activated by maximal electroconvulsive shock in hippocampus. J Neurochem. 1993;60:330–336. - PubMed
- Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82:192–201. - PubMed
- Berkeley JL, Levey AI. Cell-specific extracellular signal-regulated kinase activation by multiple G protein-coupled receptor families in hippocampus. Mol Pharmacol. 2003;63:128–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous