The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression - PubMed (original) (raw)
The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression
Chun Kim et al. Nat Immunol. 2008 Sep.
Abstract
The mitogen-activated protein kinase p38 mediates cellular responses to injurious stress and immune signaling. Among the many p38 isoforms, p38 alpha is the most widely expressed in adult tissues and can be targeted by various pharmacological inhibitors. Here we investigated how p38 alpha activation is linked to cell type-specific outputs in mouse models of cutaneous inflammation. We found that both myeloid and epithelial p38 elicit inflammatory responses, yet p38 alpha signaling in each cell type served distinct inflammatory functions and varied depending on the mode of skin irritation. In addition, myeloid p38 alpha limited acute inflammation via activation of anti-inflammatory gene expression dependent on mitogen- and stress-activated kinases. Our results suggest a dual function for p38 alpha in the regulation of inflammation and show mixed potential for its inhibition as a therapeutic strategy.
Figures
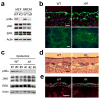
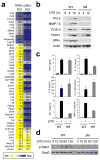
Figure 1. Disruption of Mapk14 results in efficient removal of its protein product, p38α, in myeloid and epithelial cells
(a) Whole cell lysates from Mapk14+/+ and _Mapk14_−/−MEFs (WT and KO, respectively), and WT and p38αΔM BMDMs were analyzed by immunoblotting with antibodies against the proteins indicated on the left. Data are representative of at least five independent experiments. (b) Skin (top) and spleen (bottom) tissue sections from WT and p38αΔM mice were analyzed by immunostaining with an antibody against the macrophage marker F4/80 (green). The counter staining of F-actin (red; skin) and DNA (blue; skin and spleen) is shown together. Asterisks indicate nonspecific staining of stratum corneum. Scale bar, 100 μm. Data are representative of two independent experiments. (c) Whole cell lysates from WT and p38αΔK epidermis were analyzed by immunoblotting as in (a). The numbers indicate individual animals. Data are representative of analysis of at least ten litters. (d, e) Skin tissue sections from WT and p38αΔK mice were analyzed by H&E staining (d) and immunostaining (e) with an antibody against loricrin (green). The counter staining is shown as in (b). Scale bar, 100 μm. Data are representative of at least ten (d) or two (e) independent experiments.
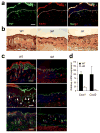
Figure 2
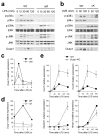
Myeloid p38α signaling is essential for chronic inflammation and acanthosis. The shaved back skin of mice was treated daily with 5% SDS for 7 days to induce chronic inflammation, and analyzed. Asterisks (a, c) indicate nonspecific staining of stratum corneum. Scale bar, 100 μm. (a) Skin section from a WT mouse was immunostained with antibodies against TNF (green; left) and F4/80 (red; middle). Merged image (right), also includes the counter stain of DNA (blue). Dotted lines denote the epidermal-dermal boundaries. Data are representative of at least three independent experiments. (b, c) Skin tissue sections from the indicated mice were analyzed by H&E staining (b), and immunostaining (c) with antibodies against K14, Ki67, and Gr-1 (green; top, middle, bottom, respectively). The counter staining of DNA (blue) and F-actin (red) is shown together. Arrowheads (white) indicate Ki67-positive nuclei. Data are representative of six independent experiments. (d) Relative mRNA expression in skin tissues (_n_=3) from the indicated mice was determined by qPCR.
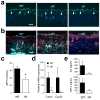
Figure 3
UVB-induced inflammatory infiltration and injury depend on epithelial p38α signaling. The shaved back skin of mice was irradiated with UVB (160 mJ/cm2) to induce epidermal injury (a–d). (a, b) Skin tissue sections from the indicated mice were analyzed by TUNEL staining 24 h post-irradiation (a) and immunostaining with Gr-1-specific antibody 96 h post-irradiation (b). Scale bar, 100 μm. Apoptotic nuclei and neutrophils (green) are shown together with the counter staining of DNA (blue) and/or F-actin (red). Arrowheads (white) indicate TUNEL-positive nuclei. Data are representative of at least three independent experiments. (c, d) Skin tissues from the indicated mice were analyzed for MPO activity (_n_=4; *P = 0.015) (c) and gene expression (_n_=3) (d) 96 h after irradiation. Relative mRNA expression was determined by qPCR. (e) Culture supernatants of the indicated keratinocytes were collected 6 h after UVB irradiation, and KC and MIP-2 concentrations were measured. Data are representative of two independent experiments.
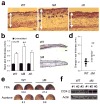
Figure 4. Myeloid p38α functions to limit edema formation in epidermal injury and irritation
(a) The shaved back skin of mice was irradiated with UVB as in Fig. 3. Skin tissue sections were prepared from the indicated mice 48 h after irradiation and analyzed by H&E staining. Double-head arrows indicate skin thickness. Scale bar, 100 μm. Data are representative of at least three independent experiments. (b) The thickness of back skin without or with UVB irradiation is measured. Data represent mean ± standard error (_n_=5; **P = 0.0015 relative to WT-UVB). (c) Skin tissue sections from the auricles of the indicated mice were prepared 24 h after TPA treatment and analyzed by H&E staining. Scale bar, 100 μm. Data are representative of analysis of five animals of each genotype. (d) Changes in ear thickness of individual mice before and after TPA treatment were determined (triangle). The horizontal bars denote the mean values in the two groups (**_P_=0.00024). (e) Evans Blue was injected intravenously into mice 6 h after treatment with TPA (right auricle) or acetone (left auricle). The auricles were photographed 30 min after dye injection. The values at the bottom of auricle images indicate relative dye leakage. Data are representative of two independent experiments. (f) Auricle skin extracts were prepared 6 h after TPA treatment and analyzed by immunoblotting with anti-COX-2 and anti-actin. Data are from one experiment involving three animals of each genotype. The numbers indicate individual animals.
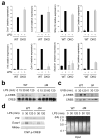
Figure 5. p38α is required for transcriptional induction of specific genes in LPS-treated macrophages
(a) Gene expression in _Ikbkb_−/−FLDMs (IKKβ-KO), and p38αΔM BMDMs (p38α KO) 4 h after treatment with LPS (100 ng/ml) was analyzed by qPCR. The values represent percentage of mRNA in these samples relative to WT samples. (b) WT and p38αΔM BMDMs were treated with LPS. At the indicated time points, whole cell lysates were prepared and analyzed by immunoblotting with antibodies against the proteins indicated on the left. PAI-2, MMP-13, COX-2, and TRAF1 are encoded by Serpinb2, Mmp13, Ptgs2, and Traf1, respectively. Data are representative of two independent experiments. (c) Culture supernatants of WT and p38αΔM BMDMs (_n_=3) were collected 12 h after LPS treatment and cytokine and chemokine concentrations were measured. (d) WT and p38αΔM BMDMs were treated with LPS and analyzed by immunoblotting as in (b). Data are representative of three independent experiments.
Figure 6. p38α signaling limits the activation of other MAP kinase signaling pathways
(a, b) Whole cell lysates from BMDMs treated with LPS (a), and keratinocytes irradiated with UVB (b) were prepared after the indicated durations of stimulation and analyzed by immunoblotting with antibodies against the proteins indicated on the left. Data are representative of at least five independent experiments. (c, d) Dusp1 mRNA expression in BMDMs (c) and keratinocytes (d) at the indicated time points following LPS treatment (c) and UVB irradiation (d) was analyzed by qPCR. Data are representative of two independent experiments. (e) Expression of genes in WT and _Dusp1_-KO BMDMs at the indicated time points following LPS treatment was analyzed by qPCR. Data are representative of two independent experiments.
Figure 7. Transcriptional induction of a subset of p38α target genes depends on MSKs
(a) Expression of genes in WT and Msk1, Msk2-DKO BMDMs at 0 and 4 h after LPS treatment was analyzed by qPCR. Data are representative of three independent experiments. (b, c) Whole cell lysates from BMDMs treated with LPS (b), and keratinocytes irradiated with UVB (c) were prepared after the indicated durations of stimulation and analyzed by immunoblotting with antibodies against phosphorylated and total CREB proteins. Data are representative of four (b) or two (c) independent experiments. (d) WT and p38αΔM BMDMs were treated with LPS for the indicated durations and analyzed by ChIP. The extent of promoter-bound phospho-CREB was determined by PCR analysis with primers specific to the indicated gene promoters. The chromatin preparations used in the immunoprecipitation (input) were separated by agarose gel electrophoresis and visualized by ethidium bromide. Data are representative of three independent experiments.
Similar articles
- Tumor necrosis factor-alpha regulates inflammatory and mesenchymal responses via mitogen-activated protein kinase kinase, p38, and nuclear factor kappaB in human endometriotic epithelial cells.
Grund EM, Kagan D, Tran CA, Zeitvogel A, Starzinski-Powitz A, Nataraja S, Palmer SS. Grund EM, et al. Mol Pharmacol. 2008 May;73(5):1394-404. doi: 10.1124/mol.107.042176. Epub 2008 Feb 5. Mol Pharmacol. 2008. PMID: 18252806 - Differential roles of JNK, ERK1/2, and p38 mitogen-activated protein kinases on endothelial cell tissue repair functions in response to tumor necrosis factor-α.
Kanaji N, Nelson A, Wang X, Sato T, Nakanishi M, Gunji Y, Basma H, Michalski J, Farid M, Rennard SI, Liu X. Kanaji N, et al. J Vasc Res. 2013;50(2):145-56. doi: 10.1159/000345525. Epub 2012 Dec 18. J Vasc Res. 2013. PMID: 23258237 - Second-generation inhibitors demonstrate the involvement of p38 mitogen-activated protein kinase in post-transcriptional modulation of inflammatory mediator production in human and rodent airways.
Birrell MA, Wong S, McCluskie K, Catley MC, Hardaker EL, Haj-Yahia S, Belvisi MG. Birrell MA, et al. J Pharmacol Exp Ther. 2006 Mar;316(3):1318-27. doi: 10.1124/jpet.105.093310. Epub 2005 Dec 20. J Pharmacol Exp Ther. 2006. PMID: 16368902 - Novel strategies for inhibition of the p38 MAPK pathway.
Zhang J, Shen B, Lin A. Zhang J, et al. Trends Pharmacol Sci. 2007 Jun;28(6):286-95. doi: 10.1016/j.tips.2007.04.008. Epub 2007 May 7. Trends Pharmacol Sci. 2007. PMID: 17482683 Review. - p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases.
Kumar S, Boehm J, Lee JC. Kumar S, et al. Nat Rev Drug Discov. 2003 Sep;2(9):717-26. doi: 10.1038/nrd1177. Nat Rev Drug Discov. 2003. PMID: 12951578 Review.
Cited by
- p38α protein negatively regulates T helper type 2 responses by orchestrating multiple T cell receptor-associated signals.
Hu P, Nebreda AR, Liu Y, Carlesso N, Kaplan M, Kapur R. Hu P, et al. J Biol Chem. 2012 Sep 28;287(40):33215-26. doi: 10.1074/jbc.M112.355594. Epub 2012 Aug 2. J Biol Chem. 2012. PMID: 22859305 Free PMC article. - Differential Production of Type I IFN Determines the Reciprocal Levels of IL-10 and Proinflammatory Cytokines Produced by C57BL/6 and BALB/c Macrophages.
Howes A, Taubert C, Blankley S, Spink N, Wu X, Graham CM, Zhao J, Saraiva M, Ricciardi-Castagnoli P, Bancroft GJ, O'Garra A. Howes A, et al. J Immunol. 2016 Oct 1;197(7):2838-53. doi: 10.4049/jimmunol.1501923. Epub 2016 Aug 22. J Immunol. 2016. PMID: 27549173 Free PMC article. - New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation.
Ariel A, Serhan CN. Ariel A, et al. Front Immunol. 2012 Jan 31;3:4. doi: 10.3389/fimmu.2012.00004. eCollection 2012. Front Immunol. 2012. PMID: 22566890 Free PMC article. - Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity.
Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M. Medders KE, et al. J Immunol. 2010 Oct 15;185(8):4883-95. doi: 10.4049/jimmunol.0902535. Epub 2010 Sep 20. J Immunol. 2010. PMID: 20855878 Free PMC article. - Three new compounds with nitric oxide inhibitory activity from Tirpitzia sinensis, an ethnomedicinal plant from Southwest China.
Gu R, Wang Y, Wu S, Wang Y, Li P, Xu L, Zhou Y, Chen Z, Kennelly EJ, Long C. Gu R, et al. BMC Chem. 2019 Apr 1;13(1):47. doi: 10.1186/s13065-019-0568-9. eCollection 2019 Dec. BMC Chem. 2019. PMID: 31384795 Free PMC article.
References
- Lee JC, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. - PubMed
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–8. - PubMed
- Rouse J, et al. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. - PubMed
- Freshney NW, et al. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. - PubMed
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_U127081014/MRC_/Medical Research Council/United Kingdom
- R01 AR045284-10/AR/NIAMS NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R01 AR045284/AR/NIAMS NIH HHS/United States
- R01 AR055218-01A1/AR/NIAMS NIH HHS/United States
- P30 DK043351-18/DK/NIDDK NIH HHS/United States
- (DK043351/DK/NIDDK NIH HHS/United States
- R01 AR055218/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases