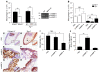Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice - PubMed (original) (raw)
Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice
Yanqing Gong et al. J Clin Invest. 2008 Sep.
Abstract
Inflammation plays a critical role in the development of cardiovascular diseases. Infiltration of leukocytes to sites of injury requires their exit from the blood and migration across basement membrane; this process has been postulated to require remodeling of the ECM. Plasminogen (Plg) is a protease that binds to the ECM and, upon conversion to plasmin, degrades multiple ECM proteins. In addition, plasmin directly activates MMPs. Here, we used Plg(-/-) mice to investigate the role of Plg in inflammatory leukocyte migration. After induction of peritonitis by thioglycollate injection, we found that Plg(-/-) mice displayed diminished macrophage trans-ECM migration and decreased MMP-9 activation. Furthermore, injection of the active form of MMP-9 in Plg(-/-) mice rescued macrophage migration in this model. We used periaortic application of CaCl2 to induce abdominal aortic aneurysm (AAA) and found that Plg(-/-) mice displayed reduced macrophage infiltration and were protected from aneurysm formation. Administration of active MMP-9 to Plg(-/-) mice promoted macrophage infiltration and the development of AAA. These data suggest that Plg regulates macrophage migration in inflammation via activation of MMP-9, which, in turn, regulates the ability of the cells to migrate across ECM. Thus, targeting the Plg/MMP-9 pathway may be an attractive approach to regulate inflammatory responses and AAA development.
Figures
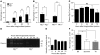
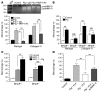
Figure 1. Plg activation is required for macrophage recruitment.
(A) Macrophage recruitment in Plg+/+ and Plg–/– mice after thioglycollate injection (n = 3–7). (B) Recruitment of macrophages is suppressed in Plg–/– mice and enhanced in PAI1–/– mice (n = 9–22 mice per group). (C) Plg in plasma of Plg+/+ mice (n = 3) is unaffected by thioglycollate treatment. (D) Plasmin (83 kDa) activity in PLF was measured by casein zymography. (E) Intensity of plasmin bands in Plg+/+ PLF (3 independent assays). (F) Aprotinin and tranexamic acid (TA) reduce thioglycollate-induced macrophage recruitment in Plg+/+ mice (n = 8–10). *P < 0.05, **P < 0.01.
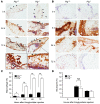
Figure 2. Trans-ECM migration of macrophages, but not neutrophils, is blocked in peritoneal tissue of Plg–/– mice.
Peritoneal tissue was dissected and examined after thioglycollate treatment. (A) Macrophage (Mac-3 antibody). Original magnification, ×100; insets, ×400. Arrowheads indicate mesothelial layer; asterisk indicates macrophage accumulation area. (B) Neutrophil (neutrophil antibody). Original magnification, ×200. (C and D) Macrophage and neutrophil distribution (expressed as percentage of tissue area) in peritoneal tissue (n = 5–6). *P < 0.05, **P < 0.01.
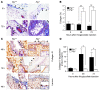
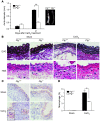
Figure 3. Collagen content is altered in thioglycollate-injected Plg–/– mice.
(A–C) Peritoneal tissue from Plg+/+ and Plg–/– mice was stained after thioglycollate treatment. (A) Collagen stained blue by Masson’s trichrome. Original magnification, ×200; insets, ×400. Arrowheads indicate mesothelial layer. (B) Total collagen as percentage of total section area (n = 4–6). **P < 0.01. (C) Collagen IV and laminin. Arrowheads indicate mesothelial layer; asterisk indicates macrophage accumulation area (n = 4–6). Original magnification, ×200; insets, ×400. (D) Collagen IV degradation. PLF of mice was isolated after thioglycollate injection, and soluble collagen IV degradation products were quantified by ELISA (n = 4). *P < 0.05.
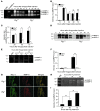
Figure 4. MMP-9 is activated by Plg in vivo and in vitro.
(A–D) MMP–9 activity. PLF was collected after thioglycollate treatment at different time points and subjected to MMP-9 activity analysis. proMMP-9 (105 kDa) and actMMP-9 (95 kDa, 88 kDa), as well as proMMP-2 (72 kDa and 69 kDa), were identified by molecular weight relative to markers and purified protein. (A) Gelatin zymography. (B and C) Quantitation of the intensity of actMMP-9 bands and the ratio of actMMP-9 to proMMP-9 bands from zymogram results (n = 3). (D) MMP-9 immunoblot. PLF was purified by gelatin-agarose and detected with MMP-9 antibody. (E and F) Peritoneal tissue extracted and analyzed by gelatin zymography. (E) Gelatin zymography. (F) Intensity of actMMP-9 bands in peritoneal tissue (n = 3). (G) Colocalization of macrophages and MMP-9 in peritoneal tissue. Tissue sections, 72 hours after thioglycollate treatment, double immunofluorescently stained for macrophages (Mac-3 antibody, green) and MMP-9 (MMP-9 antibody, red). Original magnification, ×100. Representative images taken from 3 experiments. (H) Peritoneal macrophages derived from Plg+/+ mice were isolated and cultured, which was followed by treatment with or without Plg (10 μg/ml) for 24 or 48 hours. The culture medium was collected and subjected to gelatin zymography. (I) Intensity of actMMP-9 bands in the culture medium of macrophages (3 independent assays).*P < 0.05, **P < 0.01.
Figure 5. MMP-9 activation is required for Plg-induced macrophage migration in vitro.
(A and B) Macrophage migration across Matrigel or collagen IV with serum as the chemoattractant was assayed. (A) MMP-9 neutralization blocks Plg-mediated macrophage migration. Plg+/+ macrophage migration was determined in the presence or absence of Plg, with MMP-9 antibody or control IgG. Inset: Gelatin zymography assay for culture medium of peritoneal macrophages treated with PBS, Plg plus IgG, or Plg plus MMP-9 antibody. (B) MMP-9 deficiency blocks Plg-mediated macrophage migration. Migration of Mmp9+/+ or Mmp9–/– macrophages was determined with or without Plg treatment. (C) MMP-9 knockout blocks Plg-mediated macrophage migration across Matrigel with MCP-1 as the chemoattractant. (D) Reconstitution of actMMP-9 restores macrophage migration in response to PLF from Plg–/– mice. The migration of Plg+/+ macrophages was determined in response to PLF (chemoattractant) collected from Plg+/+ and Plg–/– mice with or without actMMP-9. In A–D, “Control” indicates wells with cells and medium only. Migrated cells were counted by microscopy in 4 high-power fields for each insert (3 separate experiments performed in triplicate). **P < 0.01.
Figure 6. Macrophages require MMP-9 in vivo to migrate across peritoneal tissue, and actMMP-9 rescues impaired macrophage migration in Plg–/– mice.
(A) Macrophage migration in Mmp9+/+ or Mmp9–/– mice and in Plg+/+ mice treated with control IgG or anti–MMP-9 antibody was measured 72 hours after thioglycollate injection (n = 5–7). Inset, gelatin zymograph of PLF from Plg+/+ mice treated with IgG or MMP-9 antibody shows that the MMP-9 neutralization abolishes MMP-9 activation. (B–J) Before thioglycollate injection, mice were treated with PBS, proMMP-9, and actMMP-9, and 48 hours after thioglycollate injection, peritoneal lavage and tissue were collected. Results are from 3 independent assays (n = 5–7). (B) actMMP-9 restores the suppressed macrophage recruitment to the peritoneal cavity in Plg–/– mice. (C–H) Collagen IV (collagen IV antibody) and macrophage (Mac-3 antibody) immunostaining in Plg–/– mice. Arrowheads indicate mesothelial layer; asterisk indicates macrophage accumulation area. Original magnification (C and D), ×100; insets, ×200. Original magnification (E–H), ×200; insets, ×400. (I) Macrophage distribution expressed as percentage of total sample area in peritoneal tissue. (J) Soluble collagen IV degradation products in PLF of Plg–/– mice. *P < 0.05, **P < 0.01.
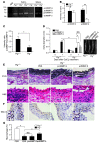
Figure 7. Prevention of AAA by Plg deficiency.
Three weeks after treatment (CaCl2 or NaCl), abdominal aorta was dissected and examined (n = 6–8). (A) Aortic diameter as measured before and after treatment (left). Representative photograph (right). (B) Aorta stained with EVG for elastic lamella (top row) and H&E for inflammatory cells (bottom row). Original magnification, ×400. (C) Macrophages (Mac-3 antibody) in aorta. EL, elastic lamellae. Original magnification, ×200; inset, ×400. (D) Macrophage distribution expressed as percentage of tissue area in aortic tissue (n = 4–5). *P < 0.05, **P < 0.01.
Figure 8. Plg-mediated AAA formation requires MMP-9.
(A–C) MMP activity in aorta 1 week after CaCl2 or NaCl (Sham) treatment. (D–G) CaCl2-treated mice were injected with proMMP-9, actMMP-9, or PBS (n = 5–7), and 1 week after treatment, the abdominal aortas were examined. (A) Extracted aorta tissue (5 μg protein) was analyzed by gelatin zymography. (B) Intensity of actMMP-9 bands in zymogram assays (3 independent assays). (C) Intensity ratio (actMMP-9/proMMP-9) of zymogram assays. (D) Aortic diameter before and after treatment (left). Representative aortas are shown (right). (E) Aorta sections stained for elastic lamellae (EVG) and inflammatory cells (H&E). Original magnification, ×400. (F) Macrophages (Mac-3 antibody). Original magnification, ×200; insets, ×400. (G) Macrophage distribution expressed as percentage of total sample area in aorta (n = 4–5). *P < 0.05, **P < 0.01.
Similar articles
- Regulation of macrophage migration by a novel plasminogen receptor Plg-R KT.
Lighvani S, Baik N, Diggs JE, Khaldoyanidi S, Parmer RJ, Miles LA. Lighvani S, et al. Blood. 2011 Nov 17;118(20):5622-30. doi: 10.1182/blood-2011-03-344242. Epub 2011 Sep 22. Blood. 2011. PMID: 21940822 Free PMC article. - Moonlighting glycolytic protein glyceraldehyde-3-phosphate dehydrogenase (GAPDH): an evolutionarily conserved plasminogen receptor on mammalian cells.
Chauhan AS, Kumar M, Chaudhary S, Patidar A, Dhiman A, Sheokand N, Malhotra H, Raje CI, Raje M. Chauhan AS, et al. FASEB J. 2017 Jun;31(6):2638-2648. doi: 10.1096/fj.201600982R. Epub 2017 Mar 15. FASEB J. 2017. PMID: 28298336 - Plasmin is not protective in experimental renal interstitial fibrosis.
Edgtton KL, Gow RM, Kelly DJ, Carmeliet P, Kitching AR. Edgtton KL, et al. Kidney Int. 2004 Jul;66(1):68-76. doi: 10.1111/j.1523-1755.2004.00707.x. Kidney Int. 2004. PMID: 15200414 - New insights into the role of Plg-RKT in macrophage recruitment.
Miles LA, Lighvani S, Baik N, Parmer CM, Khaldoyanidi S, Mueller BM, Parmer RJ. Miles LA, et al. Int Rev Cell Mol Biol. 2014;309:259-302. doi: 10.1016/B978-0-12-800255-1.00005-3. Int Rev Cell Mol Biol. 2014. PMID: 24529725 Free PMC article. Review. - Crosstalk between the plasminogen/plasmin system and inflammation resolution.
Perucci LO, Vago JP, Miles LA, Sousa LP. Perucci LO, et al. J Thromb Haemost. 2023 Oct;21(10):2666-2678. doi: 10.1016/j.jtha.2023.07.013. Epub 2023 Jul 24. J Thromb Haemost. 2023. PMID: 37495082 Free PMC article. Review.
Cited by
- Deleting fibroblast growth factor 2 in macrophages aggravates septic acute lung injury by increasing M1 polarization and inflammatory cytokine secretion.
Yi L, Chen Y, Zhang Y, Huang H, Li J, Qu Y, Weng T, Chai J. Yi L, et al. Mol Biomed. 2024 Oct 22;5(1):50. doi: 10.1186/s43556-024-00203-0. Mol Biomed. 2024. PMID: 39436561 Free PMC article. - Inflammation is a critical factor for successful regeneration of the adult zebrafish retina in response to diffuse light lesion.
Bludau O, Weber A, Bosak V, Kuscha V, Dietrich K, Hans S, Brand M. Bludau O, et al. Front Cell Dev Biol. 2024 Jul 12;12:1332347. doi: 10.3389/fcell.2024.1332347. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39071801 Free PMC article. - Single-Cell RNA Sequencing Reveals Immunomodulatory Effects of Stem Cell Factor and Granulocyte Colony-Stimulating Factor Treatment in the Brains of Aged APP/PS1 Mice.
Gardner RS, Kyle M, Hughes K, Zhao LR. Gardner RS, et al. Biomolecules. 2024 Jul 10;14(7):827. doi: 10.3390/biom14070827. Biomolecules. 2024. PMID: 39062541 Free PMC article. - The emerging role of tranexamic acid and its principal target, plasminogen, in skeletal health.
Xie W, Donat A, Jiang S, Baranowsky A, Keller J. Xie W, et al. Acta Pharm Sin B. 2024 Jul;14(7):2869-2884. doi: 10.1016/j.apsb.2024.03.033. Epub 2024 Mar 30. Acta Pharm Sin B. 2024. PMID: 39027253 Free PMC article. Review. - Using a pan-cancer atlas to investigate tumour associated macrophages as regulators of immunotherapy response.
Coulton A, Murai J, Qian D, Thakkar K, Lewis CE, Litchfield K. Coulton A, et al. Nat Commun. 2024 Jul 6;15(1):5665. doi: 10.1038/s41467-024-49885-8. Nat Commun. 2024. PMID: 38969631 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- HL17964/HL/NHLBI NIH HHS/United States
- T32 HL07914/HL/NHLBI NIH HHS/United States
- R01 HL065205/HL/NHLBI NIH HHS/United States
- R01 HL017964/HL/NHLBI NIH HHS/United States
- R01 HL078701/HL/NHLBI NIH HHS/United States
- HL078701/HL/NHLBI NIH HHS/United States
- HL65205/HL/NHLBI NIH HHS/United States
- T32 HL007914/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous