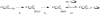The impact of enzyme engineering upon natural product glycodiversification - PubMed (original) (raw)
Review
The impact of enzyme engineering upon natural product glycodiversification
Gavin J Williams et al. Curr Opin Chem Biol. 2008 Oct.
Abstract
Glycodiversification of natural products is an effective strategy for small molecule drug development. Recently, improved methods for chemo-enzymatic synthesis of glycosyl donors has spurred the characterization of natural product glycosyltransferases (GTs), revealing that the substrate specificity of many naturally occurring GTs as too stringent for use in glycodiversification. Protein engineering of natural product GTs has emerged as an attractive approach to overcome this limitation. This review highlights recent progress in the engineering/evolution of enzymes relevant to natural product glycodiversification with a particular focus upon GTs.
Figures
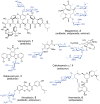
Figure 1
Natural product glycosides and their therapeutic properties. Sugar moieties are highlighted blue.
Figure 2
Chemo-enzymatic glycorandomization. E1 represents a promiscuous anomeric sugar kinase, E2 represents a promiscuous sugar-1-phosphate nucleotidyltransferase, GT represents a promiscuous glycosyltransferase and the gray oval represents a complex natural product scaffold.
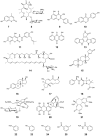
Figure 3
Engineering _N/O_-glucosylation activities in plant GTs. (a) Glycosylation of hydroxyl containing acceptors necessarily proceeds through proton abstraction whereas _N_-glycosyltransfer does not. 3,4-DCP, 3,4-dichlorophenol; 3,4-DCA, 3,4-dichloroaniline. (b) Catalytic efficiency of WT and mutant GTs toward _N_- and _O_-glucosyltransfer. WT BnUGT does not catalyze _N_-glucosyltransfer, 314N- F317Y displays considerable activity toward 3,4-DCA. The reciprocal mutations in UGT72B1 shifts the WT dual _N/O_-glucosyltransfer activity toward a more specific _O_-GT. (c) Active site of WT UGT72B1 showing the non-canonical interaction of His-19 with Ser-14. (d) Canonical geometry of the catalytic base in VvGT1 and (e) OleD (in this view, the substrate was excluded for clarity).
Figure 4
Diversity of acceptor substrates for evolved OleD variants.
Similar articles
- Natural-product sugar biosynthesis and enzymatic glycodiversification.
Thibodeaux CJ, Melançon CE 3rd, Liu HW. Thibodeaux CJ, et al. Angew Chem Int Ed Engl. 2008;47(51):9814-59. doi: 10.1002/anie.200801204. Angew Chem Int Ed Engl. 2008. PMID: 19058170 Free PMC article. Review. - Expanding the promiscuity of a natural-product glycosyltransferase by directed evolution.
Williams GJ, Zhang C, Thorson JS. Williams GJ, et al. Nat Chem Biol. 2007 Oct;3(10):657-62. doi: 10.1038/nchembio.2007.28. Epub 2007 Sep 9. Nat Chem Biol. 2007. PMID: 17828251 - Unusual sugar biosynthesis and natural product glycodiversification.
Thibodeaux CJ, Melançon CE, Liu HW. Thibodeaux CJ, et al. Nature. 2007 Apr 26;446(7139):1008-16. doi: 10.1038/nature05814. Nature. 2007. PMID: 17460661 Review. - Promiscuity Characteristics of Versatile Plant Glycosyltransferases for Natural Product Glycodiversification.
Zhang LJ, Wang DG, Zhang P, Wu C, Li YZ. Zhang LJ, et al. ACS Synth Biol. 2022 Feb 18;11(2):812-819. doi: 10.1021/acssynbio.1c00489. Epub 2022 Jan 25. ACS Synth Biol. 2022. PMID: 35076210 - Chapter 12. The power of glycosyltransferases to generate bioactive natural compounds.
Härle J, Bechthold A. Härle J, et al. Methods Enzymol. 2009;458:309-33. doi: 10.1016/S0076-6879(09)04812-5. Methods Enzymol. 2009. PMID: 19374988
Cited by
- Divergent evolution of an atypical S-adenosyl-l-methionine-dependent monooxygenase involved in anthracycline biosynthesis.
Grocholski T, Dinis P, Niiranen L, Niemi J, Metsä-Ketelä M. Grocholski T, et al. Proc Natl Acad Sci U S A. 2015 Aug 11;112(32):9866-71. doi: 10.1073/pnas.1501765112. Epub 2015 Jul 27. Proc Natl Acad Sci U S A. 2015. PMID: 26216966 Free PMC article. - Functional screening of metagenome and genome libraries for detection of novel flavonoid-modifying enzymes.
Rabausch U, Juergensen J, Ilmberger N, Böhnke S, Fischer S, Schubach B, Schulte M, Streit WR. Rabausch U, et al. Appl Environ Microbiol. 2013 Aug;79(15):4551-63. doi: 10.1128/AEM.01077-13. Epub 2013 May 17. Appl Environ Microbiol. 2013. PMID: 23686272 Free PMC article. - Glycosyltransferase engineering for carbohydrate synthesis.
McArthur JB, Chen X. McArthur JB, et al. Biochem Soc Trans. 2016 Feb;44(1):129-42. doi: 10.1042/BST20150200. Biochem Soc Trans. 2016. PMID: 26862198 Free PMC article. Review. - Engineered biosynthesis of gilvocarcin analogues with altered deoxyhexopyranose moieties.
Shepherd MD, Liu T, Méndez C, Salas JA, Rohr J. Shepherd MD, et al. Appl Environ Microbiol. 2011 Jan;77(2):435-41. doi: 10.1128/AEM.01774-10. Epub 2010 Nov 12. Appl Environ Microbiol. 2011. PMID: 21075894 Free PMC article. - Transcriptomic analysis and carbohydrate metabolism-related enzyme expression across different pH values in Rhizopus delemar.
Liang J, Chen Y, Li S, Liu D, Tian H, Xiang Q, Zhao K, Yu X, Chen Q, Fan H, Zhang L, Penttinen P, Gu Y. Liang J, et al. Front Microbiol. 2024 Mar 6;15:1359830. doi: 10.3389/fmicb.2024.1359830. eCollection 2024. Front Microbiol. 2024. PMID: 38511010 Free PMC article.
References
- Williams GJ, Zhang C, Thorson JS. Natural product glycosyltransferases: Properties and applications. Adv Enzymol Relat Areas Mol Biol. :76. in press. - PubMed
- Weymouth-Wilson AC. The role of carbohydrates in biologically active natural products. Nat Prod Rep. 1997;14:99–110. - PubMed
- Křen V, Řezanka T. Sweet antibiotics – the role of glycosidic residues in antibiotic and antitumor activity and their randomization. FEMS Microbiol Rev. 2008;32:858–889. - PubMed
- Ahmed A, Peters NR, Fitzgerald MK, Watson JA, Jr, Hoffmann FM, Thorson JS. Colchicine glycorandomization influences cytotoxicity and mechanism of action. J Am Chem Soc. 2006;128:14224–14225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources