Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel - PubMed (original) (raw)
. 2008 Aug 5;5(8):e157; discussion e157.
doi: 10.1371/journal.pmed.0050157.
Sally Sharpe, Carolina Herrera, Alethea Cope, Mike Dennis, Neil Berry, Claire Ham, Jonathan Heeney, Naser Rezk, Angela Kashuba, Peter Anton, Ian McGowan, Robin Shattock
Affiliations
- PMID: 18684007
- PMCID: PMC2494562
- DOI: 10.1371/journal.pmed.0050157
Prevention of SIV rectal transmission and priming of T cell responses in macaques after local pre-exposure application of tenofovir gel
Martin Cranage et al. PLoS Med. 2008.
Abstract
Background: The rectum is particularly vulnerable to HIV transmission having only a single protective layer of columnar epithelium overlying tissue rich in activated lymphoid cells; thus, unprotected anal intercourse in both women and men carries a higher risk of infection than other sexual routes. In the absence of effective prophylactic vaccines, increasing attention is being given to the use of microbicides and preventative antiretroviral (ARV) drugs. To prevent mucosal transmission of HIV, a microbicide/ARV should ideally act locally at and near the virus portal of entry. As part of an integrated rectal microbicide development programme, we have evaluated rectal application of the nucleotide reverse transcriptase (RT) inhibitor tenofovir (PMPA, 9-[(R)-2-(phosphonomethoxy) propyl] adenine monohydrate), a drug licensed for therapeutic use, for protective efficacy against rectal challenge with simian immunodeficiency virus (SIV) in a well-established and standardised macaque model.
Methods and findings: A total of 20 purpose-bred Indian rhesus macaques were used to evaluate the protective efficacy of topical tenofovir. Nine animals received 1% tenofovir gel per rectum up to 2 h prior to virus challenge, four macaques received placebo gel, and four macaques remained untreated. In addition, three macaques were given tenofovir gel 2 h after virus challenge. Following intrarectal instillation of 20 median rectal infectious doses (MID50) of a noncloned, virulent stock of SIVmac251/32H, all animals were analysed for virus infection, by virus isolation from peripheral blood mononuclear cells (PBMC), quantitative proviral DNA load in PBMC, plasma viral RNA (vRNA) load by sensitive quantitative competitive (qc) RT-PCR, and presence of SIV-specific serum antibodies by ELISA. We report here a significant protective effect (p = 0.003; Fisher exact probability test) wherein eight of nine macaques given tenofovir per rectum up to 2 h prior to virus challenge were protected from infection (n = 6) or had modified virus outcomes (n = 2), while all untreated macaques and three of four macaques given placebo gel were infected, as were two of three animals receiving tenofovir gel after challenge. Moreover, analysis of lymphoid tissues post mortem failed to reveal sequestration of SIV in the protected animals. We found a strong positive association between the concentration of tenofovir in the plasma 15 min after rectal application of gel and the degree of protection in the six animals challenged with virus at this time point. Moreover, colorectal explants from non-SIV challenged tenofovir-treated macaques were resistant to infection ex vivo, whereas no inhibition was seen in explants from the small intestine. Tissue-specific inhibition of infection was associated with the intracellular detection of tenofovir. Intriguingly, in the absence of seroconversion, Gag-specific gamma interferon (IFN-gamma)-secreting T cells were detected in the blood of four of seven protected animals tested, with frequencies ranging from 144 spot forming cells (SFC)/10(6) PBMC to 261 spot forming cells (SFC)/10(6) PBMC.
Conclusions: These results indicate that colorectal pretreatment with ARV drugs, such as tenofovir, has potential as a clinically relevant strategy for the prevention of HIV transmission. We conclude that plasma tenofovir concentration measured 15 min after rectal administration may serve as a surrogate indicator of protective efficacy. This may prove to be useful in the design of clinical studies. Furthermore, in vitro intestinal explants served as a model for drug distribution in vivo and susceptibility to virus infection. The finding of T cell priming following exposure to virus in the absence of overt infection is provocative. Further studies would reveal if a combined modality microbicide and vaccination strategy is feasible by determining the full extent of local immune responses induced and their protective potential.
Conflict of interest statement
Competing Interests: The authors declare no competing financial interests.
Figures
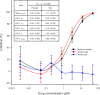
Figure 1. Solution and Gel Formulated Tenofovir Inhibited SIVmac251/32H Infectivity In Vitro at Similar Doses and in the Same Range as for Representative HIV-1 Isolates
Infection of TZM-bl indicator cells in the presence and absence of tenofovir was compared by luminescence analysis of cell lysates and the results expressed as percent inhibition. The graph shows the full titration of drug formulations on infection with SIVmac251/32H; the virus stock used in subsequent challenge experiments in vivo. Each point represents the mean of three independent experiments performed in triplicate +/− standard deviation. The results for a panel of HIV-1 strains in comparison to SIVmac251/32H are shown as IC50 values in the inset.
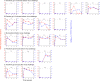
Figure 2. Rectal Administration of Tenofovir Gel Protected a High Proportion of Macaques against Subsequent Acquisition of SIV by Rectal Transmission
The results of VI from PBMC are shown as + or − for each animal. The temporal profiles of plasma vRNA concentration (red dot) and frequency of PMBC-associated proviral DNA (blue triangle) are shown for each animal in the study.
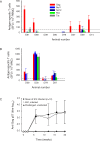
Figure 3. SIV-Specific IFN-γ Secreting T Cells Were Detected in SIV-Challenged Macaques in the Absence of Serum Antibody Responses and Evidence of Overt Infection
(A) IFN-γ secreting T cell frequencies in PBMC from protected animals (D68–D14) compared to those in an SIV-infected animal (E81) measured 20 wk after virus exposure measured by ex vivo ELISpot. The mean frequencies of three replicate determinations plus one standard deviation are shown for each peptide pool used. (B) SIV-specific IFN-γ secreting T cell frequencies in MNC isolated from ileum–jejunum tissue of four protected animals measured post mortem at 21 wk after virus challenge by ex vivo ELISpot. (C) The group mean +/− standard deviation profile of anti-SIV Gag p27 binding antibody titres (measured by ELISA) from animals infected with SIV (○) and the individual profile for an SIV-infected macaque E81 (○), in which T cell ELISpot was analysed was compared with animals from which no virus was detected following challenge (▴).
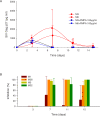
Figure 4. Colorectal Explants from Macaques Supported Replication of SIV That Was Inhibited by Pretreatment with Tenofovir In Vitro and In Vivo
(A) Replication dynamics of SIVmac251/32H in explants from two untreated animals (group F: M3, M6) in the presence or absence of exogenously added tenofovir at 100 μg/ml. A total of 104 TCID50 of virus was added to each well containing three explants in a total volume of 200 μl of medium. Virus replication was assayed by SIV Gag p27 production and mean values +/− standard deviations are shown for four replicates of each tissue. (B) Colorectal explants from four animals (group G: M1, M38, M5, M32) dosed in vivo with tenofovir per rectum 3 h before tissue removal were exposed to virus in vitro (as described above) and culture supernatants assayed for Gag p27. Mean percent inhibition of SIV Gag p27 production plus standard deviations are shown.
Comment in
- Can a topical microbicide prevent rectal HIV transmission?
Hladik F, Dezzutti CS. Hladik F, et al. PLoS Med. 2008 Aug 5;5(8):e167. doi: 10.1371/journal.pmed.0050167. PLoS Med. 2008. PMID: 18684009 Free PMC article.
Similar articles
- Pharmacokinetics of tenofovir following intravaginal and intrarectal administration of tenofovir gel to rhesus macaques.
Nuttall J, Kashuba A, Wang R, White N, Allen P, Roberts J, Romano J. Nuttall J, et al. Antimicrob Agents Chemother. 2012 Jan;56(1):103-9. doi: 10.1128/AAC.00597-11. Epub 2011 Oct 10. Antimicrob Agents Chemother. 2012. PMID: 21986823 Free PMC article. - Tenofovir treatment augments anti-viral immunity against drug-resistant SIV challenge in chronically infected rhesus macaques.
Metzner KJ, Binley JM, Gettie A, Marx P, Nixon DF, Connor RI. Metzner KJ, et al. Retrovirology. 2006 Dec 21;3:97. doi: 10.1186/1742-4690-3-97. Retrovirology. 2006. PMID: 17184540 Free PMC article. - Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir.
García-Lerma JG, Otten RA, Qari SH, Jackson E, Cong ME, Masciotra S, Luo W, Kim C, Adams DR, Monsour M, Lipscomb J, Johnson JA, Delinsky D, Schinazi RF, Janssen R, Folks TM, Heneine W. García-Lerma JG, et al. PLoS Med. 2008 Feb;5(2):e28. doi: 10.1371/journal.pmed.0050028. PLoS Med. 2008. PMID: 18254653 Free PMC article. - Rectal microbicide development.
McGowan I. McGowan I. Curr Opin HIV AIDS. 2012 Nov;7(6):526-33. doi: 10.1097/COH.0b013e3283582bc2. Curr Opin HIV AIDS. 2012. PMID: 23032732 Free PMC article. Review. - Rectal microbicide development.
McGowan I, Dezzutti C. McGowan I, et al. Curr Top Microbiol Immunol. 2014;383:117-36. doi: 10.1007/82_2013_325. Curr Top Microbiol Immunol. 2014. PMID: 23612991 Free PMC article. Review.
Cited by
- A drug evaluation of 1% tenofovir gel and tenofovir disoproxil fumarate tablets for the prevention of HIV infection.
Gengiah TN, Baxter C, Mansoor LE, Kharsany AB, Abdool Karim SS. Gengiah TN, et al. Expert Opin Investig Drugs. 2012 May;21(5):695-715. doi: 10.1517/13543784.2012.667072. Epub 2012 Mar 7. Expert Opin Investig Drugs. 2012. PMID: 22394224 Free PMC article. Review. - Reverse transcriptase inhibitors as potential colorectal microbicides.
Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. Herrera C, et al. Antimicrob Agents Chemother. 2009 May;53(5):1797-807. doi: 10.1128/AAC.01096-08. Epub 2009 Mar 2. Antimicrob Agents Chemother. 2009. PMID: 19258271 Free PMC article. - Can a topical microbicide prevent rectal HIV transmission?
Hladik F, Dezzutti CS. Hladik F, et al. PLoS Med. 2008 Aug 5;5(8):e167. doi: 10.1371/journal.pmed.0050167. PLoS Med. 2008. PMID: 18684009 Free PMC article. - Advances in HIV microbicide development.
Olsen JS, Easterhoff D, Dewhurst S. Olsen JS, et al. Future Med Chem. 2011 Dec;3(16):2101-16. doi: 10.4155/fmc.11.153. Future Med Chem. 2011. PMID: 22098355 Free PMC article. Review. - Mucosal transmission of human immunodeficiency virus.
Tebit DM, Ndembi N, Weinberg A, Quiñones-Mateu ME. Tebit DM, et al. Curr HIV Res. 2012 Jan 1;10(1):3-8. doi: 10.2174/157016212799304689. Curr HIV Res. 2012. PMID: 22264040 Free PMC article. Review.
References
- Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, et al. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150:306–311. - PubMed
- Kalichman SC, Pompa D, Luke W, Austin J. HIV transmission risk behaviours among HIV-positive persons in serodiscordant relationships. Int J STD AIDS. 2002;13:677–682. - PubMed
- Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997;146:350–357. - PubMed
- Leynaert B, Downs AM, De V I. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol. 1998;148:88–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI050410/AI/NIAID NIH HHS/United States
- U19 AI060614/AI/NIAID NIH HHS/United States
- U19 AI060614-01/AI/NIAID NIH HHS/United States
- AI50410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials