Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation - PubMed (original) (raw)
Phosphate is essential for inhibition of the mitochondrial permeability transition pore by cyclosporin A and by cyclophilin D ablation
Emy Basso et al. J Biol Chem. 2008.
Abstract
Energized mouse liver mitochondria displayed the same calcium retention capacity (a sensitive measure of the propensity of the permeability transition pore (PTP) to open) irrespective of whether phosphate, arsenate, or vanadate was the permeating anion. Unexpectedly, however, phosphate was specifically required for PTP desensitization by cyclosporin A (CsA) or by genetic inactivation of cyclophilin D (CyP-D). Indeed, when phosphate was replaced by arsenate, vanadate, or bicarbonate, the inhibitory effects of CsA and of CyP-D ablation on the PTP disappeared. After loading with the same amount of Ca(2+) in the presence of arsenate or vanadate but in the absence of phosphate, the sensitivity of the PTP to a variety of inducers was identical in mitochondria from wild-type mice, CyP-D-null mice, and wild-type mice treated with CsA. These findings call for a reassessment of conclusions on the role of the PTP in cell death that are based on the effects of CsA or of CyP-D ablation.
Figures
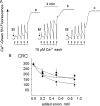
FIGURE 1.
Effect of anions on the mitochondrial permeability transition. A, the incubation medium was supplemented with 1 m
m
Asi (trace a), 1 m
m
Vi (trace b), or 1 m
m
Pi (trace c). The conditions were as follows: 2-ml final volume, pH 7.4, and 25 °C. Where indicated, 1 mg of mouse liver mitochondria (M) was added, followed by a train of Ca2+ pulses of 10 μ
m
each (arrows).B, the experimental conditions were as described for A, except that the medium contained 0.1 m
m
Pi plus the indicated concentrations of Asi (▴), Vi (▪), and Pi (•). The CRC is expressed in nanomoles of Ca2+/mg of protein.
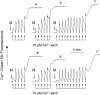
FIGURE 2.
Desensitization of the PTP by CsA or by CyP-D ablation depends on Pi. Where indicated (M), 1 mg of wild-type mitochondria treated with 0.8 μ
m
CsA (traces a–c) or of CyP-D-null mitochondria (_traces a_′_–c_′) was added to medium containing 1 m
m
Asi (traces a and _a_′), 1 m
m
Vi (traces b and _b_′), or 1 m
m
Pi (traces c and c_′) under conditions otherwise identical to those described in the legend to Fig. 1_A.
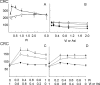
FIGURE 3.
Desensitization of the PTP by CsA or by CyP-D ablation depends on Pi. A, wild-type mitochondria treated with 0.8 μ
m
CsA (○) or CyP-D-null mitochondria (▴) were incubated in the presence of the indicated millimolar concentrations of Pi. B, wild-type (circles) or CyP-D-null (triangles) mitochondria were incubated in the presence of the indicated millimolar concentrations of Vi (open symbols) or Asi (closed symbols). C and D, wild-type mitochondria (•), CyP-D-null mitochondria (▴), or wild-type mitochondria treated with 0.8 μ
m
CsA (○) were incubated with the indicated millimolar concentrations of Pi and Vi (C) or Pi and Asi (D). The incubation conditions were otherwise identical to those described in the legend to Fig. 1_A_, and the CRC is expressed in nanomoles of Ca2+/mg of protein.
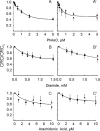
FIGURE 4.
Sensitivity of PTP to inducers is unaffected by CyP-D ablation in the absence of Pi. Wild-type (•) or CyP-D-null (▴) mitochondria were incubated in the presence of 1 m
m
Vi (A–C) or 1 m
m
Asi (A_′_–C_′) in the presence of the indicated concentrations of phenylarsine oxide (PhAsO), diamide, or arachidonic acid. Experimental conditions were otherwise identical to those described in the legend to Fig. 1_A, and the CRC is normalized to the value measured in the absence of PTP inducers (CRC0).
Similar articles
- The mitochondrial phosphate carrier interacts with cyclophilin D and may play a key role in the permeability transition.
Leung AW, Varanyuwatana P, Halestrap AP. Leung AW, et al. J Biol Chem. 2008 Sep 26;283(39):26312-23. doi: 10.1074/jbc.M805235200. Epub 2008 Jul 30. J Biol Chem. 2008. PMID: 18667415 Free PMC article. - Properties of the permeability transition pore in mitochondria devoid of Cyclophilin D.
Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Basso E, et al. J Biol Chem. 2005 May 13;280(19):18558-61. doi: 10.1074/jbc.C500089200. Epub 2005 Mar 25. J Biol Chem. 2005. PMID: 15792954 - Mitochondrial targeting of cyclosporin A enables selective inhibition of cyclophilin-D and enhanced cytoprotection after glucose and oxygen deprivation.
Malouitre S, Dube H, Selwood D, Crompton M. Malouitre S, et al. Biochem J. 2009 Dec 14;425(1):137-48. doi: 10.1042/BJ20090332. Biochem J. 2009. PMID: 19832699 Free PMC article. - Cyclophilin D as a drug target.
Waldmeier PC, Zimmermann K, Qian T, Tintelnot-Blomley M, Lemasters JJ. Waldmeier PC, et al. Curr Med Chem. 2003 Aug;10(16):1485-506. doi: 10.2174/0929867033457160. Curr Med Chem. 2003. PMID: 12871122 Review. - Genetic dissection of the permeability transition pore.
Forte M, Bernardi P. Forte M, et al. J Bioenerg Biomembr. 2005 Jun;37(3):121-8. doi: 10.1007/s10863-005-6565-9. J Bioenerg Biomembr. 2005. PMID: 16167169 Review.
Cited by
- Modulation and Pharmacology of the Mitochondrial Permeability Transition: A Journey from F-ATP Synthase to ANT.
Carrer A, Laquatra C, Tommasin L, Carraro M. Carrer A, et al. Molecules. 2021 Oct 26;26(21):6463. doi: 10.3390/molecules26216463. Molecules. 2021. PMID: 34770872 Free PMC article. Review. - Not all mitochondrial carrier proteins support permeability transition pore formation: no involvement of uncoupling protein 1.
Crichton PG, Parker N, Vidal-Puig AJ, Brand MD. Crichton PG, et al. Biosci Rep. 2009 Dec 15;30(3):187-92. doi: 10.1042/BSR20090063. Biosci Rep. 2009. PMID: 19622065 Free PMC article. - The Haves and Have-Nots: The Mitochondrial Permeability Transition Pore across Species.
Frigo E, Tommasin L, Lippe G, Carraro M, Bernardi P. Frigo E, et al. Cells. 2023 May 17;12(10):1409. doi: 10.3390/cells12101409. Cells. 2023. PMID: 37408243 Free PMC article. Review. - Two close, too close: sarcoplasmic reticulum-mitochondrial crosstalk and cardiomyocyte fate.
Dorn GW 2nd, Scorrano L. Dorn GW 2nd, et al. Circ Res. 2010 Sep 17;107(6):689-99. doi: 10.1161/CIRCRESAHA.110.225714. Circ Res. 2010. PMID: 20847324 Free PMC article. Review. - Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition.
Khairallah RJ, Sparagna GC, Khanna N, O'Shea KM, Hecker PA, Kristian T, Fiskum G, Des Rosiers C, Polster BM, Stanley WC. Khairallah RJ, et al. Biochim Biophys Acta. 2010 Aug;1797(8):1555-62. doi: 10.1016/j.bbabio.2010.05.007. Epub 2010 May 21. Biochim Biophys Acta. 2010. PMID: 20471951 Free PMC article.
References
- Kroemer, G., Galluzzi, L., and Brenner, C. (2007) Physiol. Rev. 87 99–163 - PubMed
- Bernardi, P., Krauskopf, A., Basso, E., Petronilli, V., Blachly-Dyson, E., Di Lisa, F., and Forte, M. A. (2006) FEBS J. 273 2077–2099 - PubMed
- Fournier, N., Ducet, G., and Crevat, A. (1987) J. Bioenerg. Biomembr. 19 297–303 - PubMed
- Broekemeier, K. M., Dempsey, M. E., and Pfeiffer, D. R. (1989) J. Biol. Chem. 264 7826–7830 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous