Dichotomous dopaminergic control of striatal synaptic plasticity - PubMed (original) (raw)
Dichotomous dopaminergic control of striatal synaptic plasticity
Weixing Shen et al. Science. 2008.
Abstract
At synapses between cortical pyramidal neurons and principal striatal medium spiny neurons (MSNs), postsynaptic D1 and D2 dopamine (DA) receptors are postulated to be necessary for the induction of long-term potentiation and depression, respectively-forms of plasticity thought to underlie associative learning. Because these receptors are restricted to two distinct MSN populations, this postulate demands that synaptic plasticity be unidirectional in each cell type. Using brain slices from DA receptor transgenic mice, we show that this is not the case. Rather, DA plays complementary roles in these two types of MSN to ensure that synaptic plasticity is bidirectional and Hebbian. In models of Parkinson's disease, this system is thrown out of balance, leading to unidirectional changes in plasticity that could underlie network pathology and symptoms.
Figures
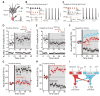
Fig. 1
D2 MSNs displayed bidirectional Hebbian STDP dependent upon D2 and A2a receptors. (A) Schematic illustration of the recording/stimulation configuration. (B) The theta-burst pairing protocols for induction of LTP and (C) LTD. (B and C) Scale bars: 40 mV × 200 ms. (D) LTP induced in by a positive timing pairing. Plots show EPSP amplitude and input resistance as a function of time. The dashed line shows the average EPSP amplitude before induction. The induction was performed at the vertical bar. Filled symbol shows the averages of 12 trials (± s.e.m.). The averaged EPSP traces before and after induction are showed at the top. Scale bars: 2 mV × 100 ms. (E) LTD induced by a negative timing pairing. Plots and EPSP traces as in (D). Scale bars: 2 mV × 100 ms. (F) In the presence of D2 receptor antagonist sulpiride (10 μM), negative timing pairing failed to alter EPSP amplitude. But co-application of A2a adenosine receptor agonist CGS 21680 (100 nM) and sulpiride led to LTP (n = 6; P < 0.05, Wilcoxon). G) LTP induction (n = 11; P < 0.01, Wilcoxon) was disrupted by the NMDA receptor antagonists APV (50 μM) and MK-801 (20 μM) or the A2a receptor antagonist SCH58261 (100 nM). (H) In the presence of D2 receptor agonist quinpirole (10 μM, n = 6), the application of the positive timing protocol leads to induction of LTD. Application of quinpirole and CGS21680 (100 nM, n = 6) together restored LTP with a positive timing pairing. (I) Schematic illustration shows that activation of A2a and NMDA receptors leads to LTP and activation of D2 and mGluR5 receptors and CaV1.3 channels leads to LTD. Moreover, A2a and D2 receptor activation opposes each other in inducing plasticity. Glu, glutamate; EC, endocannabinoid.
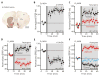
Fig. 2
D1 MSNs displayed bidirectional Hebbian STDP dependent upon D1 receptors. (A) LTP induction by a positive timing pairing protocol. EPSP amplitude and input resistance of the recorded cell were plotted as a function of time. The averaged EPSP traces before and after induction are shown at the top. Scale bars: 2 mV × 100 ms. (B) LTP induction (n = 10; P < 0.01, Wilcoxon test) was blocked by APV (50 μM) and MK-801 (20 μM). (C) LTD was not induced in D1 neurons with a negative pairing. Plots and EPSP traces are from a single cell as in (A). Scale bars: 2 mV × 100 ms. (D) In the presence of D1 receptor antagonist SCH23390 (3 μM), a negative timing pairing revealed LTD. But in the presence of CB1 receptor antagonist AM-251 (2 μM), negative pairing failed to alter EPSP amplitude. (E) LTP induced by a positive timing pairing was blocked by SCH23390, revealing LTD. LTD induced in the presence of SCH23390 was disrupted by AM-251. (F) Schematic drawing shows that activation of D1 and NMDA receptors evokes LTP and activation of mGluR5 receptor and CaV1.3 channels evokes LTD. Moreover, D1 and mGluR5 receptor activation opposes each other in inducing plasticity.
Fig. 3
Bidirectional Hebbian STDP is disrupted in MSNs from parkinsonian mice. (A) Light microscopic image of a coronal section showing the loss of immunoreactivity for TH following unilateral 6-OHDA lesioning. Cx, cortex; CPu, caudate putamen. (B) LTP was induced from lesioned D2 mice and (C) reserpine treated mice following a positive timing protocol. Plot of average EPSP amplitude as a function of time. In (C), timing-dependent LTP induced in reserpine treated animals was blocked by SCH58261 (100 nM). (D) LTP also was induced with a negative timing protocol that would normally induce LTD. In contrast, perfusion of quinpirole (10 μM) restored LTD. (E) timing-dependent LTD was evoked in D1 MSNs from lesioned D1 mice and (F) reserpine-treated mice. In (F), D1 receptor agonist SKF81297 (3 μM) restored LTP following a positive timing protocol. The LTD induced in reserpine-treated mice was disrupted by AM-251 (2 μM).
Similar articles
- Dopaminergic treatment weakens medium spiny neuron collateral inhibition in the parkinsonian striatum.
Wei W, Ding S, Zhou FM. Wei W, et al. J Neurophysiol. 2017 Mar 1;117(3):987-999. doi: 10.1152/jn.00683.2016. Epub 2016 Dec 7. J Neurophysiol. 2017. PMID: 27927785 Free PMC article. - Dopamine facilitates dendritic spine formation by cultured striatal medium spiny neurons through both D1 and D2 dopamine receptors.
Fasano C, Bourque MJ, Lapointe G, Leo D, Thibault D, Haber M, Kortleven C, Desgroseillers L, Murai KK, Trudeau LÉ. Fasano C, et al. Neuropharmacology. 2013 Apr;67:432-43. doi: 10.1016/j.neuropharm.2012.11.030. Epub 2012 Dec 8. Neuropharmacology. 2013. PMID: 23231809 - Selective Role of RGS9-2 in Regulating Retrograde Synaptic Signaling of Indirect Pathway Medium Spiny Neurons in Dorsal Striatum.
Song C, Anderson GR, Sutton LP, Dao M, Martemyanov KA. Song C, et al. J Neurosci. 2018 Aug 8;38(32):7120-7131. doi: 10.1523/JNEUROSCI.0493-18.2018. Epub 2018 Jul 13. J Neurosci. 2018. PMID: 30006367 Free PMC article. - Dopaminergic control of synaptic plasticity in the dorsal striatum.
Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Centonze D, et al. Eur J Neurosci. 2001 Mar;13(6):1071-7. doi: 10.1046/j.0953-816x.2001.01485.x. Eur J Neurosci. 2001. PMID: 11285003 Review. - D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons.
Surmeier DJ, Ding J, Day M, Wang Z, Shen W. Surmeier DJ, et al. Trends Neurosci. 2007 May;30(5):228-35. doi: 10.1016/j.tins.2007.03.008. Epub 2007 Apr 3. Trends Neurosci. 2007. PMID: 17408758 Review.
Cited by
- Using rodent data to elucidate dopaminergic mechanisms of ADHD: Implications for human personality.
Tripp G, Wickens J. Tripp G, et al. Personal Neurosci. 2024 Jan 31;7:e2. doi: 10.1017/pen.2023.12. eCollection 2024. Personal Neurosci. 2024. PMID: 38384667 Free PMC article. Review. - The chronotron: a neuron that learns to fire temporally precise spike patterns.
Florian RV. Florian RV. PLoS One. 2012;7(8):e40233. doi: 10.1371/journal.pone.0040233. Epub 2012 Aug 6. PLoS One. 2012. PMID: 22879876 Free PMC article. - Differential Dopamine Regulation of Ca(2+) Signaling and Its Timing Dependence in the Nucleus Accumbens.
Swapna I, Bondy B, Morikawa H. Swapna I, et al. Cell Rep. 2016 Apr 19;15(3):563-573. doi: 10.1016/j.celrep.2016.03.055. Epub 2016 Apr 7. Cell Rep. 2016. PMID: 27068462 Free PMC article. - Morphological elucidation of basal ganglia circuits contributing reward prediction.
Fujiyama F, Takahashi S, Karube F. Fujiyama F, et al. Front Neurosci. 2015 Feb 5;9:6. doi: 10.3389/fnins.2015.00006. eCollection 2015. Front Neurosci. 2015. PMID: 25698913 Free PMC article. Review. - Developmental Changes in Dendritic Spine Morphology in the Striatum and Their Alteration in an A53T α-Synuclein Transgenic Mouse Model of Parkinson's Disease.
Parajuli LK, Wako K, Maruo S, Kakuta S, Taguchi T, Ikuno M, Yamakado H, Takahashi R, Koike M. Parajuli LK, et al. eNeuro. 2020 Aug 27;7(4):ENEURO.0072-20.2020. doi: 10.1523/ENEURO.0072-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32817196 Free PMC article.
References
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. Science. 1994;265:1826. - PubMed
- Yin HH, Knowlton BJ. Nat Rev Neurosci. 2006;7:464. - PubMed
- Reynolds JN, Hyland BI, Wickens JR. Nature. 2001;413:67. - PubMed
- Schultz W. J Neurophysiol. 1998;80:1. - PubMed
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. Trends Neurosci. 2007;30:228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034696/NS/NINDS NIH HHS/United States
- P50 MH074866/MH/NIMH NIH HHS/United States
- NS 34696/NS/NINDS NIH HHS/United States
- MH074866/MH/NIMH NIH HHS/United States
- R37 NS034696/NS/NINDS NIH HHS/United States
- DA10044/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous