Membrane curvature induced by Arf1-GTP is essential for vesicle formation - PubMed (original) (raw)
Membrane curvature induced by Arf1-GTP is essential for vesicle formation
Rainer Beck et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The GTPase Arf1 is considered as a molecular switch that regulates binding and release of coat proteins that polymerize on membranes to form transport vesicles. Here, we show that Arf1-GTP induces positive membrane curvature and find that the small GTPase can dimerize dependent on GTP. Investigating a possible link between Arf dimerization and curvature formation, we isolated an Arf1 mutant that cannot dimerize. Although it was capable of exerting the classical role of Arf1 as a coat receptor, it could not mediate the formation of COPI vesicles from Golgi-membranes and was lethal when expressed in yeast. Strikingly, this mutant was not able to deform membranes, suggesting that GTP-induced dimerization of Arf1 is a critical step inducing membrane curvature during the formation of coated vesicles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Arf1-mediated tubulation of synthetic lipid sheets. Lipids containing p23 lipopeptide were spotted on a glass surface and hydrated with buffer containing either GTP or GTP and the exchange factor ARNO (50 nM). After addition of myristoylated Arf1-GDP (1 μM), the lipid surface was observed by light microscopy. Shown are the reactions in the presence of Arf1-wt with GTP (A), Arf1-wt with GDP (B), and Arf1-Y35A with GTP (C). Scale bars, 5 μm.
Fig. 2.
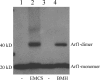
Arf1 dimerizes on protein-free liposomes. Protein-free liposomes were incubated with full-length myristoylated Arf1, GTP, and the cross-linkers EMCS (lane 2) or BMH (lane 4) as indicated. After centrifugation, the liposome-bound material was analyzed by Western blotting with an anti-Arf1 antibody.
Fig. 3.
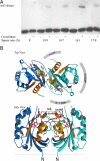
Biochemical characterization and modeling of an Arf1-GTP dimer. (A) The distance between the cysteines in an Arf1 dimer is optimal at 16Å. Protein-free liposomes were incubated with full-length myristoylated Arf1, GTPγS, and homobifunctional thiol-reactive cross-linkers of increasing spacer length as indicated (see Materials and Methods). The liposome-bound material was analyzed by Western blotting with an anti-Arf1 antibody. (B) Model of an Arf1 dimer. Shown are models of the Arf1-GTP dimer in a view toward the membrane (Upper) and from the membrane plane (Lower). The model is based on the crystal structure of monomeric Arf1-GTP (PDB ID code 1O3Y) and the constraints as described in the text. The binding site of the Arf-GAP1 catalytic domain and mapped coatomer interactions are shown schematically for one monomer. The two missing cross-linked peptides from the mass spectrometry experiment are colored in yellow (–36) and orange (152–178), and the critical tyrosyl residue in position 35, whose mutation to alanin results in a strong dimerization phenotype, is indicated. The cross-linked residues are connected by dashed lines, and their distances are indicated (the distance between Cys-159 and Lys-36 in the model is 10 Å, in accordance with the distance of the functional groups within the cross-linker EMCS). The interface coincides with the binding site of the N-terminal helix in Arf1-GDP and includes an intermolecular β-sheet completion formed by the exposed interswitch regions of Arf1-GTP.
Fig. 4.
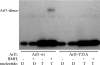
Dimerization assay on Golgi membranes. Golgi-enriched membranes from rat liver were incubated with full-length myristoylated Arf1-wt, Arf1-Y35A, and GTPγS, and after recovery of membranes by centrifugation, the cross-linker BMH was added as described in Materials and Methods. Membrane-bound material was analyzed by Western blotting with an anti-Arf1 antibody.
Fig. 5.
The non dimerizing mutant Arf1-Y35A is lethal in yeast and does not support COPI-vesicle formation in vitro. (A) In vivo analysis of Arf1 mutants in yeast. Yeast strain NYY539 (_arf1_Δ, _arf2_Δ, pURA3-Arf1) was transformed with pRS315-Arf1, pRS315-arf1-S162A, and pRS315-arf1-Y35A. Transformants were spotted in 10−1 dilution series on SDC+FOA. Pictures were taken after 2 days or 5 days of incubation at 30°C. (B) In vitro formation of COPI vesicles in the presence of monomeric or dimeric Arf1. Golgi-enriched membranes were pretreated with 250 mM KCl and incubated with purified constituents of the COPI machinery, such as Arf1-wt or the monomeric mutant Y35A, and rabbit liver coatomer and GTP. COPI-coated vesicles were purified by sucrose density gradient centrifugation. For each sample, 5% of total input (I), and 50% of the purified vesicles (V) were analyzed by SDS/PAGE and Western blotting using antibodies against the coatomer subunit δ-COP and the transmembrane protein transferrin receptor (TfR). (C) Quantification of vesicle formation in the presence of monomeric or dimeric Arf1. Vesicles were generated either as described in Materials and Methods or after isopycnic density gradient centrifugation (33), followed by negative staining electron microscopy. Twenty meshes each of the samples were randomly chosen and the number of vesicles counted.
Similar articles
- Arf1-GTP-induced tubule formation suggests a function of Arf family proteins in curvature acquisition at sites of vesicle budding.
Krauss M, Jia JY, Roux A, Beck R, Wieland FT, De Camilli P, Haucke V. Krauss M, et al. J Biol Chem. 2008 Oct 10;283(41):27717-27723. doi: 10.1074/jbc.M804528200. Epub 2008 Aug 7. J Biol Chem. 2008. PMID: 18693248 Free PMC article. - Regulation of GTP hydrolysis on ADP-ribosylation factor-1 at the Golgi membrane.
Szafer E, Rotman M, Cassel D. Szafer E, et al. J Biol Chem. 2001 Dec 21;276(51):47834-9. doi: 10.1074/jbc.M106000200. Epub 2001 Oct 9. J Biol Chem. 2001. PMID: 11592960 - Multiple and stepwise interactions between coatomer and ADP-ribosylation factor-1 (Arf1)-GTP.
Sun Z, Anderl F, Fröhlich K, Zhao L, Hanke S, Brügger B, Wieland F, Béthune J. Sun Z, et al. Traffic. 2007 May;8(5):582-93. doi: 10.1111/j.1600-0854.2007.00554.x. Traffic. 2007. PMID: 17451557 - Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation.
Antonny B, Bigay J, Casella JF, Drin G, Mesmin B, Gounon P. Antonny B, et al. Biochem Soc Trans. 2005 Aug;33(Pt 4):619-22. doi: 10.1042/BST0330619. Biochem Soc Trans. 2005. PMID: 16042557 Review. - ARF1 and SAR1 GTPases in endomembrane trafficking in plants.
Cevher-Keskin B. Cevher-Keskin B. Int J Mol Sci. 2013 Sep 5;14(9):18181-99. doi: 10.3390/ijms140918181. Int J Mol Sci. 2013. PMID: 24013371 Free PMC article. Review.
Cited by
- Shared mechanisms in physiological and pathological nucleoplasmic reticulum formation.
Drozdz MM, Vaux DJ. Drozdz MM, et al. Nucleus. 2017 Jan 2;8(1):34-45. doi: 10.1080/19491034.2016.1252893. Epub 2016 Oct 31. Nucleus. 2017. PMID: 27797635 Free PMC article. Review. - CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature.
Miller SE, Mathiasen S, Bright NA, Pierre F, Kelly BT, Kladt N, Schauss A, Merrifield CJ, Stamou D, Höning S, Owen DJ. Miller SE, et al. Dev Cell. 2015 Apr 20;33(2):163-75. doi: 10.1016/j.devcel.2015.03.002. Dev Cell. 2015. PMID: 25898166 Free PMC article. - Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans.
Cevik S, Hori Y, Kaplan OI, Kida K, Toivenon T, Foley-Fisher C, Cottell D, Katada T, Kontani K, Blacque OE. Cevik S, et al. J Cell Biol. 2010 Mar 22;188(6):953-69. doi: 10.1083/jcb.200908133. Epub 2010 Mar 15. J Cell Biol. 2010. PMID: 20231383 Free PMC article. - Differential roles of ArfGAP1, ArfGAP2, and ArfGAP3 in COPI trafficking.
Weimer C, Beck R, Eckert P, Reckmann I, Moelleken J, Brügger B, Wieland F. Weimer C, et al. J Cell Biol. 2008 Nov 17;183(4):725-35. doi: 10.1083/jcb.200806140. J Cell Biol. 2008. PMID: 19015319 Free PMC article. - Coordinated Activation of ARF1 GTPases by ARF-GEF GNOM Dimers Is Essential for Vesicle Trafficking in Arabidopsis.
Brumm S, Singh MK, Nielsen ME, Richter S, Beckmann H, Stierhof YD, Fischer AM, Kumaran M, Sundaresan V, Jürgens G. Brumm S, et al. Plant Cell. 2020 Aug;32(8):2491-2507. doi: 10.1105/tpc.20.00240. Epub 2020 Jun 2. Plant Cell. 2020. PMID: 32487565 Free PMC article.
References
- Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. - PubMed
- Serafini T, et al. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: A novel role for a GTP-binding protein. Cell. 1991;67:239–253. - PubMed
- D'Souza-Schorey C, Chavrier P. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–358. - PubMed
- Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases