Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells - PubMed (original) (raw)
. 2008 Aug 12;14(2):135-45.
doi: 10.1016/j.ccr.2008.07.003.
Tammy Ellis, Shirley L Markant, Tracy-Ann Read, Jessica D Kessler, Melissa Bourboulas, Ulrich Schüller, Robert Machold, Gord Fishell, David H Rowitch, Brandon J Wainwright, Robert J Wechsler-Reya
Affiliations
- PMID: 18691548
- PMCID: PMC2538687
- DOI: 10.1016/j.ccr.2008.07.003
Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells
Zeng-Jie Yang et al. Cancer Cell. 2008.
Abstract
Medulloblastoma is the most common malignant brain tumor in children, but the cells from which it arises remain unclear. Here we examine the origin of medulloblastoma resulting from mutations in the Sonic hedgehog (Shh) pathway. We show that activation of Shh signaling in neuronal progenitors causes medulloblastoma by 3 months of age. Shh pathway activation in stem cells promotes stem cell proliferation but only causes tumors after commitment to-and expansion of-the neuronal lineage. Notably, tumors initiated in stem cells develop more rapidly than those initiated in progenitors, with all animals succumbing by 3-4 weeks. These studies suggest that medulloblastoma can be initiated in progenitors or stem cells but that Shh-induced tumorigenesis is associated with neuronal lineage commitment.
Figures
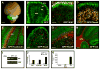
Figure 1. Conditional knockout mice allow GNP-specific deletion of ptc and activation of the hedgehog pathway
A. Brain from P8 Math1-Cre/R26R-GFP mouse shows Cre-induced GFP expression in the cerebellum. B. Cerebellar sections show GFP in the external and internal granule layers (EGL and IGL), but not in the molecular layer (ML) or white matter (WM). Within the EGL and IGL, GFP co-localizes with proliferating (Ki67+) GNPs and post-mitotic (NeuN+) GNPs and granule neurons (yellow staining in C and D). No GFP is detected in Calbindin+ Purkinje cells (pc, panel E), Pax2+ interneuron progenitors (F), BLBP+ Bergmann glia (bg) and astrocytes (G), or O4+ oligodendrocytes in the WM (H). I-J. GNPs from neonatal PtcC/C mice were infected with no virus (−) or viruses carrying GFP or Cre-IRES-GFP. Infected (GFP+) cells were FACS-sorted and mRNA analyzed by conventional RT-PCR (I) to detect ptc and beta2-microglobulin (b2M), or by real-time RT-PCR (J) to detect expression of hedgehog target genes gli1, cyclin D1 and N-myc. K. Cells infected with indicated viruses were cultured for 48h before being pulsed with tritiated thymidine (3H-Td), cultured for an additional 18h, and harvested to measure thymidine incorporation. Data represent means of triplicate samples ± SEM. Scale bars: panel A, 3mm; panels B-H, 25μm.
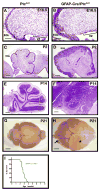
Figure 2. Deletion of ptc in GNPs leads to severe hyperplasia
Math1-Cre mice were crossed with PtcC/C mice, and brains of WT or mutant progeny were harvested at indicated ages. Brains were fixed and photographed intact (C, G) or sectioned and stained with H&E (A, B, D, E, F, H). Note the enlargement of the cerebellum and severe hyperplasia on the surface at P8 and P21. Scale bars: 30 μm (A, B, E and F); 2.5mm (C and G); 400 μm (D and H).
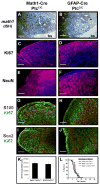
Figure 3. Most Math1-Cre/PtcC/C GNPs differentiate despite loss of ptc
A–F. Cerebella from 6-week-old WT (A, D) and Math1-Cre/PtcC/C mice (B, C, E, F) were stained with H&E (A–C) or with anti-Ki67 (green) to detect proliferation and anti-GABRA6 (red) to detect granule neuron differentiation (D–F). Whereas WT cerebella contain no proliferating cells (A, D), mutants exhibit extensive proliferation and differentiation, with proliferating cells either localized to the surface (B, E) or in nodules surrounded by differentiated cells (C, F). G–I. Cerebellar sections from 6-week-old WT (G) and Math1-Cre/PtcC/C (H) mice were stained with cresyl violet, and the regions indicated by arrows were laser-captured for RNA analysis. I. RNA from WT IGL (lane 1, derived from region 1 in panel G), Math1-Cre/PtcC/C IGL (lane 2, from region 2 in panel H), WT EGL (lane 3, tissue not shown) and proliferating cells at the surface of the Math1-Cre/PtcC/C cerebellum (lane 4, from region 4 in panel H) were analyzed by RT-PCR for expression of ptc and beta2-microglobulin (β2m). Note the lack of ptc expression in mutant IGL (lane 2). Scale bars: panels A–C, 300 μm; panels D–H, 30 μm.
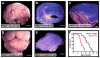
Figure 4. ptc deletion in GNPs leads to medulloblastoma
A–C. By 10 weeks of age, most Math1-Cre/PtcC/C mice develop tumors. A. Cerebellum containing tumor. B. H&E-stained section of tumor. C. H&E-stained section of secondary tumor, induced by transplantation of Math1-Cre/PtcC/C tumor cells into cerebellum of adult SCID-beige mouse. D–E. Postnatal deletion of ptc in GNPs results in medulloblastoma. Math1-CreER/PtcC/C mice were treated with tamoxifen at P4 and sacrificed when symptoms developed (in this case at 17 weeks). Cerebella were photographed intact (D) or sectioned and stained with H&E (E). F. Survival curves for Math1-Cre/PtcC/C mice (red) and Math1-CreER/PtcC/C mice treated with tamoxifen at P4 (blue). Scale bars: 3mm (A, B, D and E); 400 μm (C).
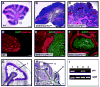
Figure 5. GFAP-Cre mice express Cre in neural stem cells
A. Cerebellar sections from E16.5 GFAP-Cre mice were stained with anti-Cre antibodies (red) and counterstained with DAPI (blue). Note the expression of Cre in the VZ but not in the EGL. B–F. Cerebellar sections from P8 GFAP-Cre/R26R-GFP mice (B–F) were stained with anti-GFP antibodies (green) to detect cells that had expressed Cre at some stage of development, and with antibodies specific for Ki67 to detect proliferating GNPs (B), NeuN to detect post-mitotic granule neurons (C), Pax2 to label interneuron progenitors (D), BLBP to label Bergmann glia (bg) and astrocytes (E) or O4 to detect oligodendrocytes in the white matter (WM, panel F). GFP was found to be co-expressed with each of these cell types (yellow staining in B–F). Scale bars represent 25 μm.
Figure 6. GFAP-Cre mediated deletion of ptc leads to expansion of NSCs
GFAP-Cre (A, C) and GFAP-Cre/PtcC/C (B, D) embryos were pulsed with BrdU two hours before being sacrificed at E14.5. Brains were harvested and cerebellar sections stained with anti-BrdU (A, B) or anti-Group B1 Sox (C, D) antibodies. Cerebella from GFAP-Cre/PtcC/C contained significantly more BrdU+ and SoxB1+ cells in the VZ. E–F. Deletion of ptc in embryonic cerebellar cells promotes increased neurosphere formation. E. Cerebella from E14.5 PtcC/C and GFAP-Cre/PtcC/C embryos were dissociated and cells were cultured at clonal density in neurosphere media. The number of neurospheres/mouse was calculated by multiplying the number of cells obtained from each embryo by the number of neurospheres observed in cultures from that embryo. Cerebella from GFAP-Cre/PtcC/C mice yielded 2.7 times more neurospheres than those from PtcC/C mice. F. Cells isolated from E14.5 PtcC/C embryos were infected with no virus or with viruses carrying GFP or Cre-IRES-GFP. Infected (GFP+) cells were sorted and cultured at clonal density in neurosphere media. After 7 days, Cre-infected cells generated 2.8 times more neurospheres than GFP-infected cells and non-infected cells. Data in E and F represent means of triplicate samples ± SEM. Scale bars: 20 μm.
Figure 7. Deletion of ptc in NSCs results in rapid tumor formation
Cerebellar sections from PtcC/C (A, C, E, G) and GFAP-Cre/PtcC/C (B, D, F, H) mice were harvested and stained with H&E (A–F) or photographed whole-mount (G–H) at E16-P21. Note the expansion of both the VZ and EGL at E16.5 (A–B) and the persistent expansion of the EGL at postnatal ages (C–F). The arrow in H points to a tumor that has formed in the cerebellum. The forebrain (asterisk) in these animals also appears enlarged; histological examination (not shown) indicates that this is due to expansion of the ventricle (perhaps due to occlusion of cerebrospinal fluid circulation) rather than to increased growth or tumorigenesis in the cortex. I. Survival curve for GFAP-Cre/PtcC/C mice. Scale bars: 20 μm (A and B); 100 μm (C and D); 300 μm (E and F); 2.5 mm (G and H).
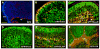
Figure 8. Tumors from Math1-Cre/PtcC/C and GFAP-Cre/PtcC/C mice display similar phenotypes
Sections from Math1-Cre/PtcC/C (A, C, E, G and I) and GFAP-Cre/PtcC/C (B, D, F, H and J) tumors were subjected to in situ hybridization to detect expression of math1 (A, B) or stained with antibodies to detect proliferating cells (Ki67, C, D, G, H, I and J), differentiating neurons (NeuN, E and F), astrocytes (S100, G and H) and stem cells (Sox2, I and J). Sections in panels C–F were counterstained with DAPI (blue). In panels A and B, asterisk denotes tumor and “bs” indicates brainstem. K. Tumor Cells were cultured for 48h, pulsed with tritiated thymidine (3H-Td), and harvested 18h later for measurement of thymidine incorporation. Data represent means of triplicate samples ± SEM. L. Survival curves of SCID-beige mice after transplantation of 1 × 106 tumor cells isolated from GFAP-Cre/PtcC/C mice, Math1-Cre/PtcC/C mice, and Math1-CreER/PtcC/C mice that had been treated with tamoxifen at P4. Scale bars represent 25 μm.
Comment in
- Even cancers want commitment: lineage identity and medulloblastoma formation.
Eberhart CG. Eberhart CG. Cancer Cell. 2008 Aug 12;14(2):105-7. doi: 10.1016/j.ccr.2008.07.011. Cancer Cell. 2008. PMID: 18691544 Free PMC article.
Similar articles
- Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma.
Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Schüller U, et al. Cancer Cell. 2008 Aug 12;14(2):123-34. doi: 10.1016/j.ccr.2008.07.005. Cancer Cell. 2008. PMID: 18691547 Free PMC article. - Even cancers want commitment: lineage identity and medulloblastoma formation.
Eberhart CG. Eberhart CG. Cancer Cell. 2008 Aug 12;14(2):105-7. doi: 10.1016/j.ccr.2008.07.011. Cancer Cell. 2008. PMID: 18691544 Free PMC article. - Serpine2/PN-1 Is Required for Proliferative Expansion of Pre-Neoplastic Lesions and Malignant Progression to Medulloblastoma.
Vaillant C, Valdivieso P, Nuciforo S, Kool M, Schwarzentruber-Schauerte A, Méreau H, Cabuy E, Lobrinus JA, Pfister S, Zuniga A, Frank S, Zeller R. Vaillant C, et al. PLoS One. 2015 Apr 22;10(4):e0124870. doi: 10.1371/journal.pone.0124870. eCollection 2015. PLoS One. 2015. PMID: 25901736 Free PMC article. - Shh signaling protects Atoh1 from degradation mediated by the E3 ubiquitin ligase Huwe1 in neural precursors.
Forget A, Bihannic L, Cigna SM, Lefevre C, Remke M, Barnat M, Dodier S, Shirvani H, Mercier A, Mensah A, Garcia M, Humbert S, Taylor MD, Lasorella A, Ayrault O. Forget A, et al. Dev Cell. 2014 Jun 23;29(6):649-61. doi: 10.1016/j.devcel.2014.05.014. Dev Cell. 2014. PMID: 24960692 - Review: personalized mice: modelling the molecular heterogeneity of medulloblastoma.
Markant SL, Wechsler-Reya RJ. Markant SL, et al. Neuropathol Appl Neurobiol. 2012 Jun;38(3):228-40. doi: 10.1111/j.1365-2990.2011.01235.x. Neuropathol Appl Neurobiol. 2012. PMID: 22070547 Review.
Cited by
- Sonic hedgehog signalling pathway in CNS tumours: its role and therapeutic implications.
Wireko AA, Ben-Jaafar A, Kong JSH, Mannan KM, Sanker V, Rosenke SL, Boye ANA, Nkrumah-Boateng PA, Poornaselvan J, Shah MH, Abdul-Rahman T, Atallah O. Wireko AA, et al. Mol Brain. 2024 Nov 20;17(1):83. doi: 10.1186/s13041-024-01155-w. Mol Brain. 2024. PMID: 39568072 Free PMC article. Review. - Understanding the autophagic functions in cancer stem cell maintenance and therapy resistance.
Niharika, Garg M. Niharika, et al. Expert Rev Mol Med. 2024 Oct 8;26:e23. doi: 10.1017/erm.2024.23. Expert Rev Mol Med. 2024. PMID: 39375840 Free PMC article. Review. - Thyroid hormone suppresses medulloblastoma progression through promoting terminal differentiation of tumor cells.
Yang Y, Valdés-Rives SA, Liu Q, Gao T, Burudpakdee C, Li Y, Tan J, Tan Y, Koch CA, Rong Y, Houser SR, Wei S, Cai KQ, Wu J, Cheng SY, Wechsler-Reya R, Yang ZJ. Yang Y, et al. Cancer Cell. 2024 Aug 12;42(8):1434-1449.e5. doi: 10.1016/j.ccell.2024.07.008. Cancer Cell. 2024. PMID: 39137728 - Permanent deconstruction of intracellular primary cilia in differentiating granule cell neurons.
Ott CM, Constable S, Nguyen TM, White K, Lee WA, Lippincott-Schwartz J, Mukhopadhyay S. Ott CM, et al. J Cell Biol. 2024 Oct 7;223(10):e202404038. doi: 10.1083/jcb.202404038. Epub 2024 Aug 13. J Cell Biol. 2024. PMID: 39137043 Free PMC article. - G-quadruplexes are a source of vulnerability in BRCA2 deficient granule cell progenitors and medulloblastoma.
Keahi DL, Sanders MA, Paul MR, Webster ALH, Fang Y, Wiley TF, Shalaby S, Carroll TS, Chandrasekharappa SC, Sandoval-Garcia C, MacMillan ML, Wagner JE, Hatten ME, Smogorzewska A. Keahi DL, et al. bioRxiv [Preprint]. 2024 Jul 22:2024.07.20.604431. doi: 10.1101/2024.07.20.604431. bioRxiv. 2024. PMID: 39091814 Free PMC article. Preprint.
References
- Adolphe C, Hetherington R, Ellis T, Wainwright B. Patched1 functions as a gatekeeper by promoting cell cycle progression. Cancer research. 2006;66:2081–2088. - PubMed
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
- Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, Katz AM, Edgar M, Kenney AM, Cordon-Cardo C, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer research. 2008;68:2241–2249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS052323-02/NS/NINDS NIH HHS/United States
- R01 NS052323-03/NS/NINDS NIH HHS/United States
- NS052323-01/NS/NINDS NIH HHS/United States
- R01 NS052323/NS/NINDS NIH HHS/United States
- R01 NS052323-01A1/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases