A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression - PubMed (original) (raw)
. 2008 Aug 12;14(2):146-55.
doi: 10.1016/j.ccr.2008.06.002.
Chiara Grisanzio, Fionnuala O'Connell, Marc Barry, Joseph M Brito, Qing Xu, Isil Guney, Raanan Berger, Paula Herman, Rachel Bikoff, Giuseppe Fedele, Won-Ki Baek, Shunyou Wang, Katharine Ellwood-Yen, Hong Wu, Charles L Sawyers, Sabina Signoretti, William C Hahn, Massimo Loda, William R Sellers
Affiliations
- PMID: 18691549
- PMCID: PMC2583442
- DOI: 10.1016/j.ccr.2008.06.002
A prostatic intraepithelial neoplasia-dependent p27 Kip1 checkpoint induces senescence and inhibits cell proliferation and cancer progression
Pradip K Majumder et al. Cancer Cell. 2008.
Abstract
Transgenic expression of activated AKT1 in the murine prostate induces prostatic intraepithelial neoplasia (PIN) that does not progress to invasive prostate cancer (CaP). In luminal epithelial cells of Akt-driven PIN, we show the concomitant induction of p27(Kip1) and senescence. Genetic ablation of p27(Kip1) led to downregulation of senescence markers and progression to cancer. In humans, p27(Kip1) and senescence markers were elevated in PIN not associated with CaP but were decreased or absent, respectively, in cancer-associated PIN and in CaP. Importantly, p27(Kip1) upregulation in mouse and human in situ lesions did not depend upon mTOR or Akt activation but was instead specifically associated with alterations in cell polarity, architecture, and adhesion molecules. These data suggest that a p27(Kip1)-driven checkpoint limits progression of PIN to CaP.
Figures
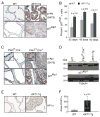
Figure 1. Induction of p27Kip1 and senescence in prostatic intraepithelial neoplasia of _AKT1_-Tg and _Pten_L/L;Cre+ mice
(A) VPs from wild-type (WT) and _AKT1_-Tg mice were stained by immunohistochemisty using antibodies directed against phospho-Akt (S473) (upper panel) or against p27Kip1 (lower panel). Scale bar, 50 μM (B) The number of p27kip1 positively staining cells was determined in the VPs of wild type (WT) and _AKT1_-Tg mice of the indicated ages. Data are presented as mean ± SEM. (C) Wild type (PtenL/L;Cre−) and Pten conditional knock out (prostate specific) (PtenL/L;Cre+) VPs were stained with phospho-Akt (S473) (upper panel) or anti-p27Kip1 (lower panel). Scale bar, 50 μM; insert 100μM (D) Western blot analysis of p27Kip1 and Tubulin in whole cell lysates from ventral prostates of Wild type (WT), _AKT1_-Tg and Pten conditional knock out (PtenL/L;Cre+) mice of 6 weeks old (E) VPs from wild type and _AKT1_-Tg mice were stained with antibody against HP1α. Scale bar,50μM. (F) Area of HP1α staining were measured both in wild type and _AKT1_-Tg prostates. Data are presented as mean ± SD.
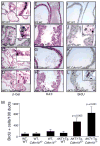
Figure 2. Genetic inactivation of Cdkn1b rescues cell from senescence and increases proliferation in _AKT1_-Tg mice
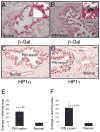
(A–L) Twelve hours after BrdU administration, mice were sacrificed and the VPs of wild-type (A, E, I), _AKT1_-Tg (B, F, J), Cdkn1b+/− (C, G, E) and _AKT1_-Tg, Cdkn1b+/− (D, H, L) mice were stained with either β-Gal (A– D), H&E (E–H) or with anti-BrdU antibody (I–L). Scale bar, 50 μM; insert 100μM. (M) The numbers of BrdU stained cells per 100 ducts was determined by manual counting of BrdU positive cells in all lobes of VP. Data are presented as mean ± SD.
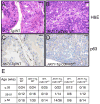
Figure 3. Genetic inactivation of Cdkn1b in _AKT1_-Tg mice results in the development of invasive prostate cancer
(A) Prostatic intraepithelial neoplasia in a representative section from the VP of _AKT1_-Tg mouse (~52 weeks old). Scale bar, 100 μM (B) Invasive prostate cancer in representative sections from _AKT1_-Tg/Cdkn1b+/−. Sections were stained with H&E. Scale bar, 100 μM (C) VP from _AKT1_-Tg mice were stained with antibodies directed against p63. Scale bar, 100 μM (D) VP from _AKT1_-Tg/Cdkn1b+/− mice were stained with antibodies directed against p63. Scale bar, 100 μM (E) Summary of tumor incidence by age and genotype.
Figure 4. Induction of p27Kip1 and increases of senescence markers do not require mTOR activation and is correlated with the PIN phenotype
(A–O) _AKT1_-Tg mice were treated with RAD001 at 10mg/kg/QD/PO for 0, 2, and 14 days (P–R) _AKT1_-Tg mice were treated with placebo for 0, 2, and 14 days. (A–C) Tissue sections from the VPs were stained with H&E, (D–F) Tissue sections were stained with antibody directed against phospho-Akt (S473) (G–I) Tissue sections were satained with antibody directed against phospho-S6RP (J–L) Immunohistochemical analysis was done in tissue sections with antibody against HP1α Scale bar, 200μM (M–R) Tissue sections were stained with antibody directed against p27Kip1. Shown are sections representative of the results obtained in at least 12 animals evaluated after each specific treatment period. Scale bar, 100 μM (A–I and M–R),
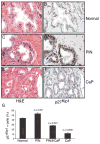
Figure 5. Stabilization of p27Kip1 in human prostate intraepithelial neoplasia
(A) Tissue sections from normal human prostate were stained with H&E (B) Normal prostate tissue sections were stained with antibody directed against p27Kip1. (C) Prostatic intraepithelial neoplasia (PIN) tissue sections were stained with H&E (D) Tissue sections from human PIN were stained with antibody directed against p27Kip1 (E) Tissue sections from human prostate cancer (CaP) were stained with H&E (F) Human prostate cancer (CaP) tissue sections were stained with antibody directed against p27Kip1. Scale bar, 50 μM (A–F). (G) The number of p27kip1 positive cells was determined in the normal, PIN, PIN adjacent to invasive cancer and CaP. Data are presented as mean ± SEM.
Figure 6. Markers of cellular senescence are elevated in human PIN
(A) Frozen sections of normal human prostate were subjected to β-Gal and hematoxylin/eosin staining. The blue staining indicates the β-Gal activity, haematoxylin and eosin was used as a counter stain (B) Frozen sections of humn Prostate Intraepithelial Neoplasia (PIN) were subjected to β-Gal and hematoxylin/eosin staining. The blue staining indicates the β-Gal activity, haematoxylin and eosin was used as a counter stain to visualize the PIN lesion (C) Tissue sections from paraffin embedded normal human prostate and PIN lesions were stained with antibodies against HP1α (D) Tissue sections from paraffin embedded normal human prostate and PIN lesions were also stained with antibodies against HP1γ. Scale bar, 50μM (A–D); insert 100 μM. (E) Average area of HP1α positive stain was measured in both normal and PIN lesion Data are presented as mean ± SD. (F) Average area of HP1γ positive stain was measured in both normal and PIN lesion Data are presented as mean ± SD.
Figure 7. Disruption of cellular polarization and adhesion is associated with upregulation of p27Kip1 level
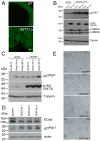
(A) Ventral prostates of both wild type (WT) and _AKT1_-Tg mice stained with Z0-1 antibody and imaged by confocal microscope. Scale bar, 50μM.. (B) Rat embryonic fibroblast cells stably transfected with either vector or Myr-AKT1 were cultured under adherent and suspension condition. Protein lysates were prepared and immunoblotted with antibodies directed against p27Kip1 (upper panel), phospho-Akt (S473), phosho-GSK3 (α and β) (middle panel) and Tubulin (lower panel). (C) Protein lysates were made from primary human epithelial cells (LEAR and LEKAR) cultured under suspension (Susp) or adherent (Ad) conditions. Immunoblot analysis using antibodies directed against p27Kip1(upper panel), phospho-Akt (S473) (middle panel), and Tubulin (lower panel) (D) Primary human epithelial cells (LEAR) were transduced with three different shRNAs against E-Cadherin and shGFP as control. Cell were harvested 2 days after post selection and total protein lysates were prepared. E-Cadherin (upper panel), p27Kip1 (middle panel) and Actin (lower panel) western blot analysis was performed (E) LEAR cells with E-Cadherin ShRNAs and control (ShGFP) were grown in plate and pictures were taken 2 days after post selection. Scale bar, 100 μM.
Similar articles
- A Prostatic Intraepithelial Neoplasia-Dependent p27Kip1 Checkpoint Induces Senescence and Inhibits Cell Proliferation and Cancer Progression.
Majumder PK, Grisanzio C, O'Connell F, Barry M, Brito JM, Xu Q, Guney I, Berger R, Herman P, Bikoff R, Fedele G, Baek WK, Wang S, Ellwood-Yen K, Wu H, Sawyers CL, Signoretti S, Hahn WC, Loda M, Sellers WR. Majumder PK, et al. Cancer Cell. 2024 Jun 10;42(6):1126. doi: 10.1016/j.ccell.2024.05.008. Epub 2024 May 30. Cancer Cell. 2024. PMID: 38866454 No abstract available. - Cooperation between FGF8b overexpression and PTEN deficiency in prostate tumorigenesis.
Zhong C, Saribekyan G, Liao CP, Cohen MB, Roy-Burman P. Zhong C, et al. Cancer Res. 2006 Feb 15;66(4):2188-94. doi: 10.1158/0008-5472.CAN-05-3440. Cancer Res. 2006. PMID: 16489020 - Expression of p27/Kip1 is down-regulated in human prostate carcinoma progression.
Fernández PL, Arce Y, Farré X, Martínez A, Nadal A, Rey MJ, Peiró N, Campo E, Cardesa A. Fernández PL, et al. J Pathol. 1999 Apr;187(5):563-6. doi: 10.1002/(SICI)1096-9896(199904)187:5<563::AID-PATH292>3.0.CO;2-3. J Pathol. 1999. PMID: 10398122 - The AMPK/p27Kip1 Pathway as a Novel Target to Promote Autophagy and Resilience in Aged Cells.
McKay LK, White JP. McKay LK, et al. Cells. 2021 Jun 8;10(6):1430. doi: 10.3390/cells10061430. Cells. 2021. PMID: 34201101 Free PMC article. Review. - Targeting p27Kip1 protein: its relevance in the therapy of human cancer.
Borriello A, Bencivenga D, Criscuolo M, Caldarelli I, Cucciolla V, Tramontano A, Borgia A, Spina A, Oliva A, Naviglio S, Della Ragione F. Borriello A, et al. Expert Opin Ther Targets. 2011 Jun;15(6):677-93. doi: 10.1517/14728222.2011.561318. Epub 2011 Feb 28. Expert Opin Ther Targets. 2011. PMID: 21355788 Review.
Cited by
- Hypoxia-inducible factor 1 is activated by dysregulated cyclin E during mammary epithelial morphogenesis.
Sengupta T, Abraham G, Xu Y, Clurman BE, Minella AC. Sengupta T, et al. Mol Cell Biol. 2011 Sep;31(18):3885-95. doi: 10.1128/MCB.05089-11. Epub 2011 Jul 11. Mol Cell Biol. 2011. PMID: 21746877 Free PMC article. - Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression.
Sprenger CC, Plymate SR, Reed MJ. Sprenger CC, et al. Int J Cancer. 2010 Dec 15;127(12):2739-48. doi: 10.1002/ijc.25615. Epub 2010 Oct 8. Int J Cancer. 2010. PMID: 21351253 Free PMC article. Review. - Activation of Akt signaling in prostate induces a TGFβ-mediated restraint on cancer progression and metastasis.
Bjerke GA, Yang CS, Frierson HF, Paschal BM, Wotton D. Bjerke GA, et al. Oncogene. 2014 Jul 10;33(28):3660-7. doi: 10.1038/onc.2013.342. Epub 2013 Sep 2. Oncogene. 2014. PMID: 23995785 Free PMC article. - PPARβ/δ promotes HRAS-induced senescence and tumor suppression by potentiating p-ERK and repressing p-AKT signaling.
Zhu B, Ferry CH, Blazanin N, Bility MT, Khozoie C, Kang BH, Glick AB, Gonzalez FJ, Peters JM. Zhu B, et al. Oncogene. 2014 Nov 13;33(46):5348-59. doi: 10.1038/onc.2013.477. Epub 2013 Nov 11. Oncogene. 2014. PMID: 24213576 Free PMC article. - A functional genetic screen defines the AKT-induced senescence signaling network.
Chan KT, Blake S, Zhu H, Kang J, Trigos AS, Madhamshettiwar PB, Diesch J, Paavolainen L, Horvath P, Hannan RD, George AJ, Sanij E, Hannan KM, Simpson KJ, Pearson RB. Chan KT, et al. Cell Death Differ. 2020 Feb;27(2):725-741. doi: 10.1038/s41418-019-0384-8. Epub 2019 Jul 8. Cell Death Differ. 2020. PMID: 31285545 Free PMC article.
References
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. - PubMed
- Berger R, Febbo PG, Majumder PK, Zhao JJ, Mukherjee S, Signoretti S, Campbell KT, Sellers WR, Roberts TM, Loda M, et al. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64:8867–8875. - PubMed
- Braig M, Lee S, Loddenkemper C, Rudolph C, Peters AH, Schlegelberger B, Stein H, Dorken B, Jenuwein T, Schmitt CA. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature. 2005;436:660–665. - PubMed
- Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01 CA094223/CA/NCI NIH HHS/United States
- P01 CA089021-06A10005/CA/NCI NIH HHS/United States
- P01 CA89021/CA/NCI NIH HHS/United States
- P01 CA089021/CA/NCI NIH HHS/United States
- K01 CA94223/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous