T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice - PubMed (original) (raw)
. 2008 Aug 22;134(4):577-86.
doi: 10.1016/j.cell.2008.06.034. Epub 2008 Aug 7.
Hong-Seok Ban, Sang-Soo Kim, Haoquan Wu, Todd Pearson, Dale L Greiner, Amale Laouar, Jiahong Yao, Viraga Haridas, Katsuyoshi Habiro, Yong-Guang Yang, Ji-Hoon Jeong, Kuen-Yong Lee, Yong-Hee Kim, Sung Wan Kim, Matthias Peipp, Georg H Fey, N Manjunath, Leonard D Shultz, Sang-Kyung Lee, Premlata Shankar
Affiliations
- PMID: 18691745
- PMCID: PMC2943428
- DOI: 10.1016/j.cell.2008.06.034
T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice
Priti Kumar et al. Cell. 2008.
Abstract
Evaluation of the therapeutic potential of RNAi for HIV infection has been hampered by the challenges of siRNA delivery and lack of suitable animal models. Using a delivery method for T cells, we show that siRNA treatment can dramatically suppress HIV infection. A CD7-specific single-chain antibody was conjugated to oligo-9-arginine peptide (scFvCD7-9R) for T cell-specific siRNA delivery in NOD/SCIDIL2rgamma-/- mice reconstituted with human lymphocytes (Hu-PBL) or CD34+ hematopoietic stem cells (Hu-HSC). In HIV-infected Hu-PBL mice, treatment with anti-CCR5 (viral coreceptor) and antiviral siRNAs complexed to scFvCD7-9R controlled viral replication and prevented the disease-associated CD4 T cell loss. This treatment also suppressed endogenous virus and restored CD4 T cell counts in mice reconstituted with HIV+ peripheral blood mononuclear cells. Moreover, scFvCD7-9R could deliver antiviral siRNAs to naive T cells in Hu-HSC mice and effectively suppress viremia in infected mice. Thus, siRNA therapy for HIV infection appears to be feasible in a preclinical animal model.
Figures
Figure 1. scFvCD7 binds to CD7 and 9R conjugation allows siRNA binding and delivery to T cells in vitro
(A) Purified human CD3+ T cells were stained with antibodies to CD3, CD4 and CD7 before or after treatment with scFvCD7Cys. (B) CD7 expression was assessed at indicated times after preincubation with scFvCD7Cys. (C) siRNA was incubated with scFvCD7-9R or unconjugated scFvCD7Cys at the indicated molar ratios for 15 min and electrophoresed on 1% agarose gels. The position of the non-bound siRNA is indicated. (D) Purified human CD3+ T cells (upper panels), CD19+ B cells (bottom panel), and differentiated CD14+ monocyte-derived macrophages (bottom panel) were treated with FITC-labeled siRNA alone (grey, filled histograms) or siRNA mixed with the indicated reagents (black, open histograms). (E) PHA-activated PBMC were treated with anti-huCD4 siRNA complexed to scFvCD7-9R. CD4 and CD8 expression levels on CD3+ T cells were monitored 60 h later (black histograms). Grey filled histograms depict control PBMC treated similarly with scFvCD7-9R/siLuc.
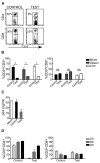
Figure 2. scFvCD7-9R-mediated siRNA uptake and gene-silencing in T cells in vivo in Hu-PBL mice
NOD/SCIDIL2rγ−/− mice reconstituted with human PBMC were injected iv with siLuc (control) or siCD4 (test) complexed to scFvCD7-9R twice, 16 h apart and human CD3+ T cells in the peripheral blood, spleen and liver were analyzed for CD4 and CD8 expression 60 h later. Representative dot plots from one mouse (A), and cumulative data from 3 mice (B), are shown. Asterisks indicate significant and “ns” indicates no significant differences between test and control groups. P < 0.05. (C) PBMC isolated from groups of Hu-PBL mice were PHA-stimulated and infected with HIVIIIB. Culture supernatants collected on day 10 after infection were tested for p24 antigen levels in triplicate by ELISA. (D) Mice were treated with siRNA 20 days after reconstitution as in (A) three times at 16 h intervals and CD4 and CD8 expression in peripheral blood T cells were determined on days 3, 6 and 9 after the last injection. Error bars indicate standard deviation.
Figure 3. iv treatment with siRNAs complexed to scFvCD7-9R prevents HIV infection in Hu-PBL mice
(A) Protocol for scFvCD7-9R/siRNA administration and immunological and virological monitoring of Hu-PBL mice infected with HIVBaL. (B-D) Hu-PBL mice were treated iv with siCCR5 or control siLuc 14 days after reconstitution. Two days later, the mice were ip infected with HIVBaL and subsequently either mock-treated (n=2) or treated with a combination of siCCR5/Vif/Tat (test, n=4) or siLuc (control, n=4) complexed to scFvCD7-9R as indicated in (A) and CD3/CD4/CD8 T cell levels were monitored by flow cytometry. Representative dot plots from one test and one control mouse are shown in (B) and cumulative data in (C). Quadrants at each time point were drawn in comparison with corresponding isotype controls. Numbers in (B) represent CD4+ or CD8+ percentages as a proportion of total CD3+ T cells. Error bars indicate standard deviations. (D) Serum p24 levels were measured by ELISA at the indicated times after viral challenge. Horizontal lines indicate median values. (E, F) Hu-PBL mice were treated with siLuc (control) or siCCR5 or siCCR5/Vif/Tat combination (siTRIPLE) complexed to scFvCD7-9R as in (A) and CD4+ T cell ratios and plasma p24 levels tested as above.
Figure 4. iv treatment with siRNA/scFvCD7-9R complexes prevents CD4 T cell loss and HIV-1 amplification in mice reconstituted with HIV-seropositive donor PBMC
(A) Protocol for siRNA/scFvCD7-9R administration and immunological and virological monitoring. (B, C) Mice transplanted with PBMC from a HIV-seropositive donor were treated iv with scFvCD7-9R complexed to either siLuc (control) or siCCR5/Vif/Tat (test) as indicated in (A) and CD4 T cell levels were monitored by flow cytometry. Representative dot plots from one mouse in each group are shown in (B) and cumulative data from 4 mice in (C). Numbers indicated in (B) represent CD4+ percentages as a proportion of total CD3+ T cells. (D) Viral copy numbers in plasma were measured by the Amplicor test on day 17 after reconstitution with donor PBMC. Error bars represent standard deviation.
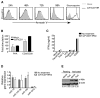
Figure 5. scFvCD7-9R mediates siRNA delivery to naïve T cells in Hu-HSC mice and suppresses HIV replication in vivo
(A) Peripheral blood from Hu-HSC mice was examined for the presence of human CD4 and CD8 T cells 12 weeks after reconstitution. (B, C) Hu-HSC mice were iv-injected twice, 16 h apart, with siCD4 (test) or control siLuc complexed to scFvCD7-9R and peripheral blood T cells were tested for CD4 and CD8 expression before and 3 days after treatment. Representative dot plots from one mouse in each group are shown in (B) and cumulative data from 3 mice in (C). Numbers indicated in (B) represent the percentage of total CD3+ T cells. In (C) the reduction in surface CD4 or CD8 levels was calculated as a percentage of the initial expression level before siRNA injection. (D) Splenocytes isolated from Hu-HSC mice 1 day after a single injection with scFvCD7-9R/siLuc (control) or siCCR5 (test) were examined for CCR5 mRNA levels by qPCR using β-actin mRNA levels for normalization. (E) Splenocytes in (D) were PHA-stimulated and infected with HIVBaL at a moi of 3 and p24 antigen levels in culture supernatants were assayed in triplicate by ELISA at the indicated time points. Error bars indicate standard deviation. (F) Hu-HSC mice were either mock-treated (n=3), treated iv with siLuc (Control, n=3) or siVif/Tat (Test, n=4) complexed to scFvCD7-9R 22 weeks after reconstitution. 18 h later, the control and test animals were ip infected with HIVBaL and further treated with scFv/siRNA every 4–5 days. Viral copy numbers in plasma measured by the Amplicor test (upper panel) and CD4+CD3+ T cell percentages monitored by flow cytometry (lower panel) at various times are shown. The grey dotted line in the upper panel represents the limit of detection of the Amplicor test. CD4 T cell ratios were calculated as a ratio of the entire CD3 population (CD4+CD3+:CD3+) and mean ratios (horizontal grey bars) at 40 d post challenge is shown. Individual animals in each group are represented by distinct symbols.
Figure 6. scFvCD7-9R/siRNA treatment does not induce toxicity
(A) scFvCD7-9R/siLuc-treated or mock-treated PBMC stimulated with PHA were stained with Annexin-V on 4 consecutive days of culture. 24 h staurosporine-treated cultures served as positive control. (B) PBMC treated with scFvCD7-9R/siLuc were stimulated with PHA or antiCD3/CD28 beads for 3 days and pulsed with 3H-thymidine for 18 h. Fold stimulation was calculated by dividing the counts incorporated in the presence of to those in the absence of stimulating agent. (C) Purified human CD4+ T cells were stimulated with anti-CD3 mAb in the absence (no treatment) or presence of Pam3CSK4 (TLR2 ligand), Poly I:C (TLR3), LPS (TLR4), Flagellin (TLR5), CLO97 (TLR8/9) or anti-CD3/CD28 Dynabeads. IFN-γ was quantified by ELISA in 48 h culture supernatants. Error bars indicate standard deviation of triplicate cultures. (D) Expression profiles of miRNA in CD3+ T cells purified from Hu-HSC mice treated thrice with scFvCD7-9R/siCCR5 are depicted. Expression level was normalized to that of small non-coding RNA U6B. Mean of triplicate runs with two animals each ± SD is shown. (E) CD3+ T cells purified from Hu-HSC mice treated as in (D) were examined for c-Myb protein levels either immediately or after 48 h in culture with PHA. The numbers below represent the ratios of band intensities of c-Myb normalized to that of β-actin. Error bars in all cases represent standard deviation.
Comment in
- Silencing HIV-1 In Vivo.
Kirchhoff F. Kirchhoff F. Cell. 2008 Aug 22;134(4):566-8. doi: 10.1016/j.cell.2008.08.004. Cell. 2008. PMID: 18724929
Similar articles
- Receptor-targeted aptamer-siRNA conjugate-directed transcriptional regulation of HIV-1.
Zhou J, Lazar D, Li H, Xia X, Satheesan S, Charlins P, O'Mealy D, Akkina R, Saayman S, Weinberg MS, Rossi JJ, Morris KV. Zhou J, et al. Theranostics. 2018 Feb 7;8(6):1575-1590. doi: 10.7150/thno.23085. eCollection 2018. Theranostics. 2018. PMID: 29556342 Free PMC article. - Silencing HIV-1 In Vivo.
Kirchhoff F. Kirchhoff F. Cell. 2008 Aug 22;134(4):566-8. doi: 10.1016/j.cell.2008.08.004. Cell. 2008. PMID: 18724929 - The human HIV/peripheral blood lymphocyte (PBL)-SCID mouse. A modified human PBL-SCID model for the study of HIV pathogenesis and therapy.
Boyle MJ, Connors M, Flanigan ME, Geiger SP, Ford H Jr, Baseler M, Adelsberger J, Davey RT Jr, Lane HC. Boyle MJ, et al. J Immunol. 1995 Jun 15;154(12):6612-23. J Immunol. 1995. PMID: 7759895 - HIV-1-specific RNA interference.
Boden D, Pusch O, Ramratnam B. Boden D, et al. Curr Opin Mol Ther. 2004 Aug;6(4):373-80. Curr Opin Mol Ther. 2004. PMID: 15468596 Review. - Current humanized mouse models for studying human immunology and HIV-1 immuno-pathogenesis.
Zhang L, Meissner E, Chen J, Su L. Zhang L, et al. Sci China Life Sci. 2010 Feb;53(2):195-203. doi: 10.1007/s11427-010-0059-7. Epub 2010 Mar 7. Sci China Life Sci. 2010. PMID: 20596827 Free PMC article. Review.
Cited by
- Functional peptides for siRNA delivery.
Tai W, Gao X. Tai W, et al. Adv Drug Deliv Rev. 2017 Feb;110-111:157-168. doi: 10.1016/j.addr.2016.08.004. Epub 2016 Aug 13. Adv Drug Deliv Rev. 2017. PMID: 27530388 Free PMC article. Review. - Lung endothelial HO-1 targeting in vivo using lentiviral miRNA regulates apoptosis and autophagy during oxidant injury.
Zhang Y, Jiang G, Sauler M, Lee PJ. Zhang Y, et al. FASEB J. 2013 Oct;27(10):4041-58. doi: 10.1096/fj.13-231225. Epub 2013 Jun 14. FASEB J. 2013. PMID: 23771928 Free PMC article. - Use of Hu-PBL Mice to Study Pathogenesis of Human-Restricted Viruses.
Brunetti JE, Kitsera M, Muñoz-Fontela C, Rodríguez E. Brunetti JE, et al. Viruses. 2023 Jan 13;15(1):228. doi: 10.3390/v15010228. Viruses. 2023. PMID: 36680271 Free PMC article. Review. - RNA interference as a novel treatment strategy for chronic hepatitis B infection.
Hui RW, Mak LY, Seto WK, Yuen MF. Hui RW, et al. Clin Mol Hepatol. 2022 Jul;28(3):408-424. doi: 10.3350/cmh.2022.0012. Epub 2022 Feb 17. Clin Mol Hepatol. 2022. PMID: 35172540 Free PMC article. Review. - Effective inhibition of hepatitis E virus replication in A549 cells and piglets by RNA interference (RNAi) targeting RNA-dependent RNA polymerase.
Huang F, Hua X, Yang S, Yuan C, Zhang W. Huang F, et al. Antiviral Res. 2009 Sep;83(3):274-81. doi: 10.1016/j.antiviral.2009.06.008. Epub 2009 Jul 1. Antiviral Res. 2009. PMID: 19576249 Free PMC article.
References
- Anderson J, Li MJ, Palmer B, Remling L, Li S, Yam P, Yee JK, Rossi J, Zaia J, Akkina R. Safety and efficacy of a lentiviral vector containing three anti-HIV genes--CCR5 ribozyme, tat-rev siRNA, and TAR decoy--in SCID-hu mouse-derived T cells. Mol Ther. 2007;15:1182–1188. - PubMed
- Banerjea A, Li MJ, Bauer G, Remling L, Lee NS, Rossi J, Akkina R. Inhibition of HIV-1 by Lentiviral Vector-Transduced siRNAs in T Lymphocytes Differentiated in SCID-hu Mice and CD34+ Progenitor Cell-Derived Macrophages. Mol Ther. 2003;8:62–71. - PubMed
- Bonilla FA, Kokron CM, Swinton P, Geha RS. Targeted gene disruption of murine CD7. Int Immunol. 1997;9:1875–1883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI071882/AI/NIAID NIH HHS/United States
- R01 A1071882/PHS HHS/United States
- UO1 AI075419/AI/NIAID NIH HHS/United States
- P30 CA034196/CA/NCI NIH HHS/United States
- R21 AI06532/AI/NIAID NIH HHS/United States
- P30 A060354/PHS HHS/United States
- CA34196/CA/NCI NIH HHS/United States
- U01 AI075419/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials