Evolutionary ecology of pungency in wild chilies - PubMed (original) (raw)
Evolutionary ecology of pungency in wild chilies
Joshua J Tewksbury et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The primary function of fruit is to attract animals that disperse viable seeds, but the nutritional rewards that attract beneficial consumers also attract consumers that kill seeds instead of dispersing them. Many of these unwanted consumers are microbes, and microbial defense is commonly invoked to explain the bitter, distasteful, occasionally toxic chemicals found in many ripe fruits. This explanation has been criticized, however, due to a lack of evidence that microbial consumers influence fruit chemistry in wild populations. In the present study, we use wild chilies to show that chemical defense of ripe fruit reflects variation in the risk of microbial attack. Capsaicinoids are the chemicals responsible for the well known pungency of chili fruits. Capsicum chacoense is naturally polymorphic for the production of capsaicinoids and displays geographic variation in the proportion of individual plants in a population that produce capsaicinoids. We show that this variation is directly linked to variation in the damage caused by a fungal pathogen of chili seeds. We find that Fusarium fungus is the primary cause of predispersal chili seed mortality, and we experimentally demonstrate that capsaicinoids protect chili seeds from Fusarium. Further, foraging by hemipteran insects facilitates the entry of Fusarium into fruits, and we show that variation in hemipteran foraging pressure among chili populations predicts the proportion of plants in a population producing capsaicinoids. These results suggest that the pungency in chilies may be an adaptive response to selection by a microbial pathogen, supporting the influence of microbial consumers on fruit chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
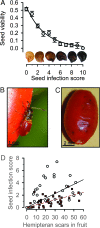
Fig. 1.
Fitness impacts and mechanics of fungal infection. (A) Seed survival (proportion of seeds germinating or still viable at end of germination trials ± 1 SE) as a function of Fusarium infection score (survival = 0.53−0.31 × fungal score, _r_2 = 0.97, P < 0.0005; n = 3414 seeds). Fusarium seed infection was scored from 0 (no infection) to 10 (uniformly black on both sides of the seed) using the seed standard pictured above the abscissa. (B) Acroleucus coxalis (Stäl) (Lygaeidae) nymph, the most common hemipteran foraging on chilies, piercing a chili fruit. (C) Ripe fruit with fungal infection spreading under surface of fruit at holes (hemipteran foraging scars). (D) Mean infection score on seeds in mature fruit as a function of hemipteran foraging scars on each fruit. Open symbols = nonpungent fruits; dark red symbols = pungent fruits. Regression forced through the origin (nonpungent _F_1,26 = 29, B = 0.075, P < 0.001; pungent _F_1,35 = 80, B = 0.041, P < 0.001).
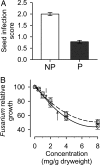
Fig. 2.
Effects of capsaicinoids on Fusarium infection. (A) Seed infection scores (mean ± 1 SE) in fruit from nonpungent (white) and pungent (red) plants (n = 10 pungent and 10 nonpungent per year). On each plant we evaluated five random fruits each year. Seed infection scores were assigned using the standard series shown in Fig. 1_A_. (B) Relative growth rate of Fusarium as a function of capsaicin (circles, solid line) and dihydrocapsaicin (squares, dashed line) concentrations. All experiments were conducted in media that mimicked the nutritional composition of wild chili fruits. Growth was measured relative to growth in a control media that lacked capsaicinoids (± 1 SE, four isolates, six replicates per isolate per treatment). Lines are quadratic functions of capsaicinoid concentration (_r_2 > 0.99, P < 0.0005). Mean capsaicin and dihydrocapsaicin concentrations in fruit at our primary study, where we assessed fungal loads, were 2.85 mg/g dry mass (solid up arrow), and 1.43 mg/g dry mass (dashed down arrow), respectively.
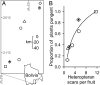
Fig. 3.
Heteropteran foraging, fungal pressure, and capsaicinoid production. (A) Locations of the seven populations in which we examined the relationship between hemipteran foraging frequency and fruit chemistry. (B) Hemipteran foraging frequency on chili fruits, an index of fusarium infection pressure, is a strong nonlinear predictor of the proportion of plants producing capsaicinoids (y = a_ln_x + b, _r_2 = 0.91, _F_1,5 = 39, P = 0.0008).
Similar articles
- A field test of the directed deterrence hypothesis in two species of wild chili.
Levey DJ, Tewksbury JJ, Cipollini ML, Carlo TA. Levey DJ, et al. Oecologia. 2006 Nov;150(1):61-8. doi: 10.1007/s00442-006-0496-y. Epub 2006 Aug 8. Oecologia. 2006. PMID: 16896774 - Why are not all chilies hot? A trade-off limits pungency.
Haak DC, McGinnis LA, Levey DJ, Tewksbury JJ. Haak DC, et al. Proc Biol Sci. 2012 May 22;279(1735):2012-7. doi: 10.1098/rspb.2011.2091. Epub 2011 Dec 21. Proc Biol Sci. 2012. PMID: 22189403 Free PMC article. - Fungal Seed Pathogens of Wild Chili Peppers Possess Multiple Mechanisms To Tolerate Capsaicinoids.
Adams CA, Zimmerman K, Fenstermacher K, Thompson MG, Skyrud W, Behie S, Pringle A. Adams CA, et al. Appl Environ Microbiol. 2020 Jan 21;86(3):e01697-19. doi: 10.1128/AEM.01697-19. Print 2020 Jan 21. Appl Environ Microbiol. 2020. PMID: 31732572 Free PMC article. - Capsaicinoids: Pungency beyond Capsicum.
Naves ER, de Ávila Silva L, Sulpice R, Araújo WL, Nunes-Nesi A, Peres LEP, Zsögön A. Naves ER, et al. Trends Plant Sci. 2019 Feb;24(2):109-120. doi: 10.1016/j.tplants.2018.11.001. Epub 2019 Jan 7. Trends Plant Sci. 2019. PMID: 30630668 Review. - Biochemistry and molecular biology of capsaicinoid biosynthesis: recent advances and perspectives.
Arce-Rodríguez ML, Ochoa-Alejo N. Arce-Rodríguez ML, et al. Plant Cell Rep. 2019 Sep;38(9):1017-1030. doi: 10.1007/s00299-019-02406-0. Epub 2019 Apr 2. Plant Cell Rep. 2019. PMID: 30941502 Review.
Cited by
- Bacillus species are core microbiota of resistant maize cultivars that induce host metabolic defense against corn stalk rot.
Xia X, Wei Q, Wu H, Chen X, Xiao C, Ye Y, Liu C, Yu H, Guo Y, Sun W, Liu W. Xia X, et al. Microbiome. 2024 Aug 23;12(1):156. doi: 10.1186/s40168-024-01887-w. Microbiome. 2024. PMID: 39180084 Free PMC article. - Monograph of wild and cultivated chili peppers (Capsicum L., Solanaceae).
Barboza GE, García CC, Bianchetti LB, Romero MV, Scaldaferro M. Barboza GE, et al. PhytoKeys. 2022 Jun 14;200:1-423. doi: 10.3897/phytokeys.200.71667. eCollection 2022. PhytoKeys. 2022. PMID: 36762372 Free PMC article. - Patterns of secondary metabolite allocation to fruits and seeds in Piper reticulatum.
Whitehead SR, Jeffrey CS, Leonard MD, Dodson CD, Dyer LA, Bowers MD. Whitehead SR, et al. J Chem Ecol. 2013 Dec;39(11-12):1373-84. doi: 10.1007/s10886-013-0362-4. Epub 2013 Nov 6. J Chem Ecol. 2013. PMID: 24190024 - Integrating TRPV1 Receptor Function with Capsaicin Psychophysics.
Smutzer G, Devassy RK. Smutzer G, et al. Adv Pharmacol Sci. 2016;2016:1512457. doi: 10.1155/2016/1512457. Epub 2016 Jan 14. Adv Pharmacol Sci. 2016. PMID: 26884754 Free PMC article. Review. - Responses to transient receptor potential (TRP) channel agonists in Chlamydomonas reinhardtii.
Wada M, Kaizuka I, Yoshimura K. Wada M, et al. Biol Open. 2020 Jul 8;9(7):bio053140. doi: 10.1242/bio.053140. Biol Open. 2020. PMID: 32641289 Free PMC article.
References
- Tiffney BH. Seed size, dispersal syndromes, and the rise of the angiosperms: Evidence and hypothesis. Ann Miss Bot Garden. 1984;71:551–576.
- Smith JF. High species diversity in fleshy-fruited tropical understory plants. Am Nat. 2001;157:646–653. - PubMed
- Tiffney BH. Vertebrate dispersal of seed plants through time. Ann Rev Ecol Evol Syst. 2004;35:1–29.
- Herrera CM. Defense of ripe fruit from pests: Its significance in relation to plant-disperser interactions. Am Nat. 1982;120:218–241.
- Janzen DH. Seed-eaters versus seed size number toxicity and dispersal. Evolution. 1969;23:1–27. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources