Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines - PubMed (original) (raw)
Neural mechanisms of paroxysmal atrial fibrillation and paroxysmal atrial tachycardia in ambulatory canines
Alex Y Tan et al. Circulation. 2008.
Abstract
Background: The relationship between autonomic activation and the mechanisms of paroxysmal atrial fibrillation remains unclear.
Methods and results: We implanted a pacemaker and a radio transmitter in 7 dogs (group 1). After baseline recording, we paced the left atrium at 20 Hz for 1 week and then monitored left stellate ganglion nerve activity, left vagal nerve activity, and left atrial electrogram without pacing for 24 hours. This protocol repeated itself until sustained atrial fibrillation (>48 hours) was induced in 3+/-1 weeks. In another 6 dogs (group 2), we cryoablated left and right stellate ganglia and the cardiac branch of the left vagal nerve during the first surgery and then repeated the same pacing protocol until sustained atrial fibrillation was induced in 7+/-4 weeks (P=0.01). There were 4+/-2 episodes of paroxysmal atrial fibrillation per day and 10+/-3 episodes of paroxysmal atrial tachycardia per day in group 1. Simultaneous sympathovagal discharges were observed to immediately precede the onset of atrial arrhythmias in 73% of episodes. In comparison, group 2 dogs had no paroxysmal atrial fibrillation (P=0.046) or paroxysmal atrial tachycardia (P<0.001) episodes. Nerve sprouting, sympathetic hyperinnervation, and a massive elevation of transcardiac norepinephrine levels occurred in both groups.
Conclusions: Intermittent rapid left atrial pacing results in sympathetic hyperinnervation, paroxysmal atrial fibrillation, and paroxysmal atrial tachycardia. Simultaneous sympathovagal discharges are common triggers of these arrhythmias. Cryoablation of extrinsic sympathovagal nerves eliminated paroxysmal atrial fibrillation and paroxysmal atrial tachycardia, which suggests that simultaneous sympathovagal discharges and these arrhythmias are causally related. Because cryoablation only delayed but did not prevent sustained atrial fibrillation, autonomic nerve activity is not the only factor that determines atrial fibrillation maintenance.
Figures
Figure 1
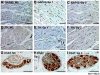
LSG and superior cardiac branch of left thoracic vagal nerve. Left edge is cranial and right edge is caudal for all panels. (A): LSG before ablation. (B): Cryoablation of LSG. (C): The left thoracic vagal nerve runs parallel to left phrenic nerve. The superior cardiac branch of the left thoracic vagal nerve is located between these two large nerves (arrow in insert). (D): Superior cardiac branch (arrow) of left vagal nerve was lifted up by the cryoprobe and cryoablated. (E-H): Histology of cryoablated SG. (E) and (F): trichrome stains of RSG, showing fibrosis at the site of ablation (arrows). (G and H): Surviving ganglion cells within the LSG staining positive (brown) for TH.
Figure 2
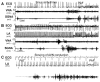
Paroxysmal atrial arrhythmias in Group 1. (A) Circadian incidence of paroxysmal arrhythmias (PAC, PAT and PAF combined) over a 24-hr period. (B): Arrow points to the PAC. (C): PAT induced by simultaneous sympathovagal discharge.
Figure 3
Two examples of PAF. (A) Sinus rhythm to AF conversion. (B) Atrial tachycardia to AF conversion. (C) Magnified from the center of Panel B (line segment above ECG), showing that the elevated VNA accelerated atrial rate, leading to paroxysmal reduction of ventricular rate (prolonged RR interval) before conversion to AF.
Figure 4
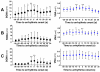
Distinctive patterns of left SGNA (black point) and VNA (blue point) 30-sec before and after the onset of PAT and PAF (A), PAC (B) and sinus tachycardia (C) in group 1 dogs, with the time of onset as time zero. There was no significant difference between PAT versus PAF, hence the data were combined. *, p<0.05 vs -30s; **, p<0.01 vs -30s; †, p<0.05 vs -5s
Figure 5
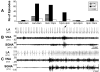
Effects of cryoablation on upstream ANA, mean RR and SDRR on postoperative days (POD) 1 and 10.
Figure 6
Effects of ANA on heart rate in noncryoablated dogs (A) and cryoablated (B, C) dogs. (A) VNA activity (a) is associated with sinus arrhythmia. SGNA burst (b) and VNA withdrawal (c) were associated with sinus tachycardia in a noncryoablated dog. (B) No or little change of atrial rate with either VNA (d) or SGNA bursts (e) in a cryoablated dog. (C) Large burst of SGNA (f) was associated with atrial rate acceleration in a cryoablated dog.
Figure 7
Upregulation of transcardiac NGF (A) and NE (B) in cryoablated (Group 2) and noncryoablated (Group 1) dogs. Repeated measures analysis of variance (rmANOVA) was performed to examine the effects of group, baseline/pacing, and rest/LSG stimulation on transcardiac NE. The rmANOVA included two-way and three-way interactions between the three factors and accounted for the four measurements from each dog, and allowed different variances for the measurements.
Figure 8
Histological sections of TH, GAP43 and ChAT atrial nerves in normal control, noncryoablated (Group 1) and cryoablated (Group 2) dogs. Both Group 1 and Group 2 dogs have significant nerve sprouting and sympathetic hyperinnervation but parasympathetic nerve densities were not significantly different from normal control.
Similar articles
- Intrinsic cardiac nerve activity and paroxysmal atrial tachyarrhythmia in ambulatory dogs.
Choi EK, Shen MJ, Han S, Kim D, Hwang S, Sayfo S, Piccirillo G, Frick K, Fishbein MC, Hwang C, Lin SF, Chen PS. Choi EK, et al. Circulation. 2010 Jun 22;121(24):2615-23. doi: 10.1161/CIRCULATIONAHA.109.919829. Epub 2010 Jun 7. Circulation. 2010. PMID: 20529998 Free PMC article. - Cryoablation of stellate ganglia and atrial arrhythmia in ambulatory dogs with pacing-induced heart failure.
Ogawa M, Tan AY, Song J, Kobayashi K, Fishbein MC, Lin SF, Chen LS, Chen PS. Ogawa M, et al. Heart Rhythm. 2009 Dec;6(12):1772-9. doi: 10.1016/j.hrthm.2009.08.011. Epub 2009 Aug 13. Heart Rhythm. 2009. PMID: 19959128 Free PMC article. - Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines.
Shen MJ, Shinohara T, Park HW, Frick K, Ice DS, Choi EK, Han S, Maruyama M, Sharma R, Shen C, Fishbein MC, Chen LS, Lopshire JC, Zipes DP, Lin SF, Chen PS. Shen MJ, et al. Circulation. 2011 May 24;123(20):2204-12. doi: 10.1161/CIRCULATIONAHA.111.018028. Epub 2011 May 9. Circulation. 2011. PMID: 21555706 Free PMC article. - Neural mechanisms of atrial arrhythmias.
Shen MJ, Choi EK, Tan AY, Lin SF, Fishbein MC, Chen LS, Chen PS. Shen MJ, et al. Nat Rev Cardiol. 2011 Sep 27;9(1):30-9. doi: 10.1038/nrcardio.2011.139. Nat Rev Cardiol. 2011. PMID: 21946776 Review. - Neural mechanisms of atrial fibrillation.
Park HW, Shen MJ, Lin SF, Fishbein MC, Chen LS, Chen PS. Park HW, et al. Curr Opin Cardiol. 2012 Jan;27(1):24-8. doi: 10.1097/HCO.0b013e32834dc4e8. Curr Opin Cardiol. 2012. PMID: 22139702 Free PMC article. Review.
Cited by
- Neurocardiac Axis Physiology and Clinical Applications.
Plott C, Harb T, Arvanitis M, Gerstenblith G, Blumenthal R, Leucker T. Plott C, et al. Int J Cardiol Heart Vasc. 2024 Aug 14;54:101488. doi: 10.1016/j.ijcha.2024.101488. eCollection 2024 Oct. Int J Cardiol Heart Vasc. 2024. PMID: 39224460 Free PMC article. Review. - Aging-associated atrial fibrillation: A comprehensive review focusing on the potential mechanisms.
Wang MF, Hou C, Jia F, Zhong CH, Xue C, Li JJ. Wang MF, et al. Aging Cell. 2024 Oct;23(10):e14309. doi: 10.1111/acel.14309. Epub 2024 Aug 12. Aging Cell. 2024. PMID: 39135295 Free PMC article. Review. - ECG-based estimation of respiration-induced autonomic modulation of AV nodal conduction during atrial fibrillation.
Plappert F, Engström G, Platonov PG, Wallman M, Sandberg F. Plappert F, et al. Front Physiol. 2024 May 8;15:1281343. doi: 10.3389/fphys.2024.1281343. eCollection 2024. Front Physiol. 2024. PMID: 38779321 Free PMC article. - Sympathetic toggled paroxysmal atrial fibrillation and recurrent premature atrial contractions in ambulatory patients.
Hwang D, Liu X, Kote A, Reaso J, Andersson KT, Shehata MM, Ehdaie A, Wang X, Cingolani E, Ramireddy A, Braunstein ED, Chen LS, Li X, Goldhaber JI, Chen PS. Hwang D, et al. Heart Rhythm. 2024 Sep;21(9):1669-1676. doi: 10.1016/j.hrthm.2024.05.029. Epub 2024 May 16. Heart Rhythm. 2024. PMID: 38762134 - Non-invasive Neuromodulation of Arrhythmias.
Rast J, Sohinki D, Warner A. Rast J, et al. J Innov Card Rhythm Manag. 2024 Feb 15;15(2):5757-5766. doi: 10.19102/icrm.2024.15022. eCollection 2024 Feb. J Innov Card Rhythm Manag. 2024. PMID: 38444451 Free PMC article. Review.
References
- Coumel P. Autonomic influences in atrial tachyarrhythmias. J Cardiovasc Electrophysiol. 1996;7:999–1007. - PubMed
- Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–9. - PubMed
- Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–60. - PubMed
- Patterson E, Po SS, Scherlag BJ, Lazzara R. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–31. - PubMed
- Sharifov OF, Fedorov VV, Beloshapko GG, Glukhov AV, Yushmanova AV, Rosenshtraukh LV. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL078931-020003/HL/NHLBI NIH HHS/United States
- R01 HL078932-02/HL/NHLBI NIH HHS/United States
- P01 HL078931-030003/HL/NHLBI NIH HHS/United States
- P01 HL078931/HL/NHLBI NIH HHS/United States
- R01 HL078932-01/HL/NHLBI NIH HHS/United States
- R01 HL078932-03/HL/NHLBI NIH HHS/United States
- R01 HL078932-04/HL/NHLBI NIH HHS/United States
- R01 HL58533/HL/NHLBI NIH HHS/United States
- R01 HL071140-07/HL/NHLBI NIH HHS/United States
- R01 HL078932/HL/NHLBI NIH HHS/United States
- R01 HL071140-06/HL/NHLBI NIH HHS/United States
- R01 HL071140/HL/NHLBI NIH HHS/United States
- R01 HL71140/HL/NHLBI NIH HHS/United States
- P01 HL078931-050003/HL/NHLBI NIH HHS/United States
- R01 HL071140-05A1/HL/NHLBI NIH HHS/United States
- R01 HL071140-06S1/HL/NHLBI NIH HHS/United States
- P01 HL078931-040003/HL/NHLBI NIH HHS/United States
- P01 HL078931-01A10003/HL/NHLBI NIH HHS/United States
- P01 HL78931/HL/NHLBI NIH HHS/United States
- R01 HL78932/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical