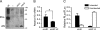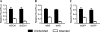Minimizing variables among hairpin-based RNAi vectors reveals the potency of shRNAs - PubMed (original) (raw)
Minimizing variables among hairpin-based RNAi vectors reveals the potency of shRNAs
Ryan L Boudreau et al. RNA. 2008 Sep.
Abstract
RNA interference (RNAi) is a cellular process regulating gene expression and participating in innate defense in many organisms. RNAi has also been utilized as a tool to query gene function and is being developed as a therapeutic strategy for several diseases. Synthetic small interfering (siRNAs) or expressed stem-loop RNAs (short-hairpin RNAs [shRNAs] or artificial microRNAs [miRNAs]) have been delivered to cultured cells and organisms to inhibit expression of a variety of genes. A persistent question in the field, however, is which RNAi expression system is most suitable for distinct applications. To date, shRNA- and artificial miRNA-based strategies have been compared with conflicting results. In prior comparisons, sequences required for efficient RNAi processing and loading of the intended antisense strand into the RNAi-induced silencing complex (RISC) were not considered. We therefore revisited the shRNA-miRNA comparison question. Initially, we developed an improved artificial miRNA vector and confirmed the optimal shRNA configuration by altering structural features of these RNAi substrates. Subsequently, we engineered and compared shRNA- and miRNA-based RNAi expression vectors that would be processed to yield similar siRNAs that exhibit comparable strand biasing. Our results demonstrate that when comparison variables are minimized, the shRNAs tested were more potent than the artificial miRNAs in mediating gene silencing independent of target sequence and experimental setting (in vitro and in vivo). In addition, we show that shRNAs are expressed at considerably higher levels relative to artificial miRNAs, thus providing mechanistic insight to explain their increased potency.
Figures
FIGURE 1.
Optimization of the human miR-30 shuttle. (A) General structures of shRNAs and artificial miRNAs (N's correspond to the siRNA-duplex region with sense and antisense being 5′ and 3′, respectively). Here, the antisense sequences are designed to target SCA1, HD, or GFP transcripts. Hash marks indicate the known major Drosha and Dicer cleavage sites of human miR-30 (Lee et al. 2003; Zeng and Cullen 2003; Silva et al. 2005). Processing sites of many shRNAs are unknown and vary based on the presence of short flanking sequences. (*) Boxed sequence is for orientation purposes in the panel. (B) Artificial miRNA variants were generated by altering the nearby flanking sequences, and portions of the predicted mFOLD (Zuker 2003) structures within the stem-base are shown. Instability (i.e., single-stranded nature) within the gray-shaded region may promote Drosha binding and cleavage (Zeng and Cullen 2005; Han et al. 2006). These variants contain identical RNAi sequences and structures in regions above the gray-shaded box. (C) Cartoon depicting RNAi reporters, generated by inserting target sequences into the 3′ UTR of Renilla luciferase. Reporter plasmids also contain a Firefly luciferase expression cassette for normalization. (D) Silencing activity of miR-30 variants. Artificial miSCA1 variants and RNAi reporter expression plasmids were cotransfected into HEK293 cells, and Dual-Glo Luciferase assays were performed after 24 h. Results of two independent experiments (each n = 3) are shown as mean±SEM relative to mock-treated (i.e., promoter only) controls and demonstrate that variant 2 (miV2) is the most effective artificial miRNA (even more effective than human miR-30, ** = P < 0.01). (E) Small transcript Northern blot was performed at 48 h post-transfection of miRNA variant expression plasmids into HEK293 cells. Results show that miV2 yields more mature antisense RNA (SCA1 AS) compared to the other artificial miRNA variants including the natural miR-30 shuttle.
FIGURE 2.
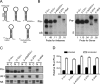
Disparate strand biasing confounds shRNA and artificial miRNA comparisons. (A) Small transcript Northern blot performed at 48 h post-transfection of HD2.1 RNAi expression plasmids in HEK293 cells shows improved yield of processed antisense RNA (AS) from CMV-driven artificial miRNA variants 1 and 2 (miV1, miV2) relative to the U6-driven first-generation shHD2.1 and a mock-treated sample (–). Pre- designates the precursor stem–loop. (B) Q-PCR analysis for endogenous HD mRNA levels performed 48 h after transfection of HD2.1 RNAi expression plasmids into HEK293 cells. Results were normalized to GAPDH mRNA levels and are shown as mean±SEM relative to mock-treated samples (n =3, * = P < 0.05). (C) Strand biasing of U6-driven HD2.1 RNAi vectors. Strand biasing was assessed by measuring luciferase activity from reporters containing either sense (intended; binds RNAi antisense) or antisense (unintended; binds RNAi sense) target sequences in the 3′ UTR (Fig. 1C). RNAi reporter and RNAi expression plasmids were cotransfected into HEK293 cells, and Dual-Glo Luciferase assays were performed at 24 h. Results are shown as mean±SEM (n = 4) relative to mock-treated controls and demonstrate that shHD2.1 preferentially loads the unintended siRNA strand while miHD2.1 more often loads the intended strand.
FIGURE 3.
shRNA processing and silencing efficiency is overhang dependent. (A) Diagrams depicting the various 5′ and 3′ overhangs tested on identical shRNA stem–loops. The 5′-27 nt (Silva et al. 2005) and 5′-XbaI (Boden et al. 2004) variants were used in prior shRNA and miRNA comparison studies. (B) Plasmids expressing the shRNA variants were transfected into HEK293 cells, and small transcript Northern blot (probing for antisense [AS] or sense [S] sequences) with densitometry analysis (values for AS and S bands are shown below blots) was performed 48 h later to assess shRNA processing efficiency (n = 3). Results show that 5′ overhang variants yield less precursor (Pre-) and antisense (AS) RNAs compared to the optimized shRNAs with U2–4 3′ overhangs (derived from Pol-III transcription termination (Ng et al. 1979; Ohshima et al. 1981; Kunkel et al. 1986). Appropriate strand loading was observed for each shRNA variant (i.e., AS:Pre->S:Pre-). (C) Plasmids expressing the shRNA variants were transfected into HEK293 cells, and nuclear/cytoplasmic fractionation and RNA isolation was performed 24 h later. Equal amounts of nuclear and cytoplasmic RNAs for each treatment group were analyzed by small transcript Northern blot (probing for antisense sequences) to evaluate where precursor (Pre-) shRNAs accumulate. Results demonstrate that the two most highly expressed shRNAs (3′-U2–4 or 3′-CU2–4) accumulate in both nuclear and cytoplasmic fractions, suggesting that saturation of both Exportin-5 and Dicer may occur. Conversely, buildup of the other shRNA variants was present primarily in the nucleus, suggesting lack of export due to suboptimal overhangs. Note: the presumed Drosha-cleavage product of the 5′-27 nt shRNA is exported and accumulates slightly in both compartments. The blot was stripped and reprobed for the nuclear U6 splicing RNA to control for fractionation and loading. Nonspecific bands (NS) present following the control probing are shown as additional fractionation and loading controls. (D) Silencing of intended (solid bars) or unintended (empty bars) target strands was assessed by cotransfection of shRNA-variant and RNAi luciferase reporter expression plasmids into HEK293 cells, and Dual Glo Luciferase assays were performed at 24 h. Results are shown as mean±SD (n = 3) relative to mock-treated controls and confirm that suboptimal overhangs decrease silencing efficiency. Notably, each shRNA preferentially silenced the intended target (transfected at 1:20 RNAi:target) relative to the unintended target (transfected at 3:1 RNAi:target).
FIGURE 4.
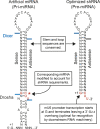
Design of comparable shRNA and artificial miRNA hairpins. Diagram depicting the design of hairpins for a fair comparison scheme. Relevant cleavage sites mapped by 3′-RACE are shown (Table 1). Vectors were designed to contain siRNAs targeting SCA1, HD, or GFP transcripts (Ns).
FIGURE 5.
Comparable shRNA- and miRNA-based vectors exhibit appropriate strand biasing. (A–C) Strand biasing of SCA1, HD, and GFP RNAi vectors, respectively. Strand biasing was assessed using luciferase reporters containing either sense (intended) or antisense (unintended) target sequences. RNAi luciferase reporter and RNAi expression plasmids were cotransfected into HEK293 cells, and Dual-Glo Luciferase assays were performed at 24 h. Results of duplicate experiments (each n = 3) are shown as mean±SEM relative to mock-treated controls.
FIGURE 6.
shRNAs are more potent than artificial miRNAs in vitro. (A) RNAi and RNAi luciferase reporter plasmids were cotransfected into HEK293 cells to assess gene silencing. Dual Glo Luciferase assays were performed at 24 h and results, shown as mean±SEM relative to mock-treated controls, were compiled from several experiments (4 GFP, 4 SCA1, and 2 HD; each n = 3). Dose is indicated as RNAi:target. P < 0.001 and P < 0.05 for 1:1 and 3:1 doses, respectively. (B,C) Plasmids expressing RNAi targeting SCA1 or HD were transfected into HEK293 cells, and Q-PCR analysis was performed at 48 h to measure reduction of endogenous transcripts. SCA1 and HD mRNA levels were normalized to GAPDH mRNA or 18S rRNA and are shown as mean±SEM (n ≥ 3, * = P < 0.05, *** = P < 0.001) relative to mock-treated controls. (D) GFP RNAi and eGFP expression plasmids were cotransfected into HEK293 cells, and fluorescence levels were evaluated 48 h later. Results are shown as mean±SEM (n = 4, ** = P < 0.01) relative to SCA1 RNAi-treated controls. (E) shRNA and artificial miRNA expression plasmids were transfected into HEK293 cells, and small transcript Northern blot was performed at 48 h to assess RNAi expression and processing. (Pre-) precursor; (AS) antisense RNA. Results show that shRNAs yield more than fourfold mature antisense RNA, relative to artificial miRNAs, independent of RNAi target sequence. These results were consistent among triplicate blots for each RNAi vector pair.
FIGURE 7.
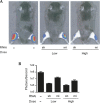
shRNAs are more potent than artificial miRNAs in vivo. (A) Gene silencing efficacy in vivo was compared by coelectroporating SCA1 RNAi and RNAi luciferase reporter plasmids into tibialis anterior muscles of 6–8-wk-old mice. Low and high doses are 1:1 and 10:1 (RNAi:target) ratios, respectively. Renilla luciferase activity was measured in vivo using bioluminescence imaging after 8 d. Representative “heat-map” images are shown along with quantitative analysis (B) represented as mean±SEM (n =4; P < 0.05 within each dose). Similar silencing trends were also observed at 4 d post-treatment (data not shown).
Similar articles
- pSM155 and pSM30 vectors for miRNA and shRNA expression.
Wu J, Bonsra AN, Du G. Wu J, et al. Methods Mol Biol. 2009;487:205-19. doi: 10.1007/978-1-60327-547-7_10. Methods Mol Biol. 2009. PMID: 19301649 - Artificial miRNAs as therapeutic tools: Challenges and opportunities.
Kotowska-Zimmer A, Pewinska M, Olejniczak M. Kotowska-Zimmer A, et al. Wiley Interdiscip Rev RNA. 2021 Jul;12(4):e1640. doi: 10.1002/wrna.1640. Epub 2021 Jan 1. Wiley Interdiscip Rev RNA. 2021. PMID: 33386705 Review. - A quick and efficient approach for gene silencing by using triple putative microRNA-based short hairpin RNAs.
Shan ZX, Lin QX, Yang M, Deng CY, Kuang SJ, Zhou ZL, Xiao DZ, Liu XY, Lin SG, Yu XY. Shan ZX, et al. Mol Cell Biochem. 2009 Mar;323(1-2):81-9. doi: 10.1007/s11010-008-9966-3. Epub 2008 Nov 27. Mol Cell Biochem. 2009. PMID: 19037714 - miRNA and shRNA expression vectors based on mRNA and miRNA processing.
Wu P, Wilmarth MA, Zhang F, Du G. Wu P, et al. Methods Mol Biol. 2013;936:195-207. doi: 10.1007/978-1-62703-083-0_16. Methods Mol Biol. 2013. PMID: 23007510 - Recombinant AAV as a platform for translating the therapeutic potential of RNA interference.
Borel F, Kay MA, Mueller C. Borel F, et al. Mol Ther. 2014 Apr;22(4):692-701. doi: 10.1038/mt.2013.285. Epub 2013 Dec 19. Mol Ther. 2014. PMID: 24352214 Free PMC article. Review.
Cited by
- MicroRNAs: short non-coding players in cancer chemoresistance.
Donzelli S, Mori F, Biagioni F, Bellissimo T, Pulito C, Muti P, Strano S, Blandino G. Donzelli S, et al. Mol Cell Ther. 2014 Jun 1;2:16. doi: 10.1186/2052-8426-2-16. eCollection 2014. Mol Cell Ther. 2014. PMID: 26056584 Free PMC article. Review. - Prospect of induced pluripotent stem cell genetic repair to cure genetic diseases.
Adiwinata Pawitan J. Adiwinata Pawitan J. Stem Cells Int. 2012;2012:498197. doi: 10.1155/2012/498197. Epub 2012 Feb 12. Stem Cells Int. 2012. PMID: 22448173 Free PMC article. - Targeting alpha-synuclein with a microRNA-embedded silencing vector in the rat substantia nigra: positive and negative effects.
Khodr CE, Becerra A, Han Y, Bohn MC. Khodr CE, et al. Brain Res. 2014 Mar 6;1550:47-60. doi: 10.1016/j.brainres.2014.01.010. Epub 2014 Jan 21. Brain Res. 2014. PMID: 24463035 Free PMC article. - Therapeutic impact of systemic AAV-mediated RNA interference in a mouse model of myotonic dystrophy.
Bisset DR, Stepniak-Konieczna EA, Zavaljevski M, Wei J, Carter GT, Weiss MD, Chamberlain JR. Bisset DR, et al. Hum Mol Genet. 2015 Sep 1;24(17):4971-83. doi: 10.1093/hmg/ddv219. Epub 2015 Jun 16. Hum Mol Genet. 2015. PMID: 26082468 Free PMC article. - The effects of stem length and core placement on shRNA activity.
McIntyre GJ, Yu YH, Lomas M, Fanning GC. McIntyre GJ, et al. BMC Mol Biol. 2011 Aug 8;12:34. doi: 10.1186/1471-2199-12-34. BMC Mol Biol. 2011. PMID: 21819628 Free PMC article.
References
- Birmingham A., Anderson E.M., Reynolds A., Ilsley-Tyree D., Leake D., Fedorov Y., Baskerville S., Maksimova E., Robinson K., Karpilow J., et al. 3′ UTR seed matches, but not overall identity, are associated with RNAi off-targets. Nat. Methods. 2006;3:199–204. - PubMed
- Bridge A.J., Pebernard S., Ducraux A., Nicoulaz A.L., Iggo R. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 2003;34:263–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD-44093/HD/NICHD NIH HHS/United States
- T32 HL007121/HL/NHLBI NIH HHS/United States
- P01 NS050210/NS/NINDS NIH HHS/United States
- DK-54759/DK/NIDDK NIH HHS/United States
- NS-50210/NS/NINDS NIH HHS/United States
- R01 HD044093/HD/NICHD NIH HHS/United States
- P30 DK054759/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources