Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging - PubMed (original) (raw)
Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging
Lisa M Guerrettaz et al. Proc Natl Acad Sci U S A. 2008.
Abstract
Aging is associated with an inability to mount protective antibody responses to vaccines and infectious agents. This decline is associated with acquisition of defects in multiple cellular compartments, including B cells. While peripheral B-cell numbers do not decline with aging, the composition of the compartment appears to change, with loss of naïve follicular B cells, accumulation of antigen-experienced cells, and alteration of the antibody repertoire. The underlying cause of this change is unknown. We tested the hypothesis that aging-associated repertoire changes can be attributed directly to decreased B lymphopoiesis. Using an Ig transgenic model to report changes in the B-cell repertoire, we show that the reduced B-cell generative capacity of "aged" long-term reconstituting hematopoietic stem cells (LT-HSCs) alters the representation of antigen specificities in the peripheral B-cell repertoire. Further, we show that reconstitution using suboptimal numbers of fully functional LT-HSCs results in the generation of a similarly altered B-cell repertoire. This may be an important factor to consider when deciding the number of bone marrow cells to transplant in the clinical setting. In conclusion, when B lymphopoiesis is limited peripheral B-cell homeostasis is altered. This is reflected in reduced diversity of the B-cell repertoire, which likely reduces the protective quality of the immune response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
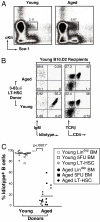
Aging-associated decline in B lymphopoiesis is B-cell/stem cell autonomous. Young or aged WT (B10.D2) mice were lethally irradiated, and lymphocyte-depleted bone marrow cells from young or prescreened immunologically aged 3-83μδ animals were transferred by i.v. injection. Donor BM was pooled from multiple animals before depletion, and BM equivalents were transferred at a ratio of one donor equivalent to four recipients. Reconstitution of the peripheral B-cell compartment was assessed 6 weeks posttransfer. Splenocytes were gated on live, CD19+ B cells, and 3-83μδ idiotype and total IgM expression was evaluated. Data are representative of analyses of a minimum of three individual animals in each category.
Fig. 2.
LT-HSCs from aged animals exhibit reduced B-cell generative capacity associated with repertoire alteration. Untreated whole BM was harvested from four young or four aged mice, RBCs were lysed, and LT-HSCs purified as described in experimental procedures. (A) Sorting profile of LT-HSCs from young and aged 3-83μδ lineage-depleted BM. (B) Reconstitution of young WT recipients 10 weeks posttransfer of 5000 LT-HSCs (Lin−Sca-1+cKit+Flk-2−CD34−) from young or aged 3-38μδ plus 3 × 105 Rag2−/− whole BM carrier cells. Total cells recovered were equivalent among recipients. Shown on the left are cytograms demonstrating IgM and 3-83μδ idiotype expression by CD19+ pregated splenocytes (B-cell plots). Shown on the right are cytograms illustrating T-cell antigen receptor (TCRβ) and CD4 expression by total splenocytes. Data are representative of analyses of a minimum of four individual animals in each category. (C) Summary of the percentage of idiotype-negative B cells present in the spleens of animals reconstituted with lineage-depleted (Fig. 1), 5-FU-treated, and flow-purified LT-HSC preparations (Fig. 2 A and B) derived from either young or aged donors. Means (horizontal bars) and statistically significant P < 0.0001* values are indicated.
Fig. 3.
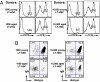
Limitation of HSC function in young mice reduces total splenic B-cell numbers and alters the B-cell repertoire. (A) BM was harvested from 5-FU-treated young 3-83μδ mice and RBC lysed. Cells were then pooled, serially diluted (represented as BM equivalents) in PBS, and each dilution spiked with 3 × 105 whole BM carriers from Rag2−/− mice. Cell mixtures were transferred to lethally irradiated young WT recipients by i.v. injection. Six weeks posttransfer, live CD19+ splenic B cells were analyzed in terms of absolute number recovered and 3-83 μδ idiotype and IgM expression. BM equivalents transferred are indicated below each bar in the graphs and _P_-values provided. Statistics were generated from a minimum of three mice in each category, error bars represent SEM. _P_-values were determined by comparing the 1:10 dilution to each subsequent dilution in turn using the _t_-test: two samples assuming equal variances. (B) Effect of anti-IL-7 inhibition of B lymphopoiesis on repertoires development in young lethally irradiated WT mice that were reconstituted with stem cells from 5-FU-treated young 3-83 μδ mice. Control mice were sham treated by injection of PBS. Splenocytes were analyzed after the last antibody injection (6 weeks posttransfer) for 3-83 μδ idiotype and IgM expression. Splenocytes were pregated on live, CD19+ cells. Data are representative of analyses of a minimum of four individual animals in each category.
Fig. 4.
Reconstitution with increasing numbers of aged LT-HSCs increases B-cell genesis and partially restores a naive peripheral repertoire. Young WT (B10.D2) mice were lethally irradiated and reconstituted with 1000 or 10,000 LT-HSCs (Lin−Sca-1+cKit+Flk-2−CD34−) sorted from young or aged 3-83 μδ donors plus 3 × 105 whole BM carriers from Rag2−/− mice. Reconstitution was assessed at 10 weeks posttransfer. (A) Histograms show frequency of CD19+ B cells and TCRβ+ T cells relative to total splenocytes recovered from recipient mice. Histogram regions containing stained populations are marked with a bar and percentages within that region are indicated. (B) Cytograms showing 3-83 μδ idiotype and IgM expression (gated on CD19+ splenocytes). Percentages of B cells falling into each quadrant are indicated. Data are representative of analyses of a minimum of four individual animals in each category.
References
- LeMaoult J, Szabo P, Weksler M E. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 1997;160:115–126. - PubMed
- Bell DR, Van Zant G. Stem cells, aging, and cancer: Inevitabilities and outcomes. Oncogene. 2004;23:7290–7296. - PubMed
- Nicoletti C, Borghesi-Nicoletti C, Yang XH, Schulze DH, Cerny J. Repertoire diversity of antibody response to bacterial antigens in aged mice. II. Phosphorylcholine-antibody in young and aged mice differ in both VH/VL gene repertoire and in specificity. J Immunol. 1991;147:2750–2755. - PubMed
- Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. - PubMed
- Weksler ME. Changes in the B-cell repertoire with age. Vaccine. 2000;18:1624–1628. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical